Figures & data
Figure 1 Study design and treatment schedule.
Abbreviations: BCVA, best-corrected visual acuity; D, Day; ICGA, indocyanine green angiography; OCT, optical coherence tomography; PRN, pro re nata; vPDT, verteporfin photodynamic therapy.
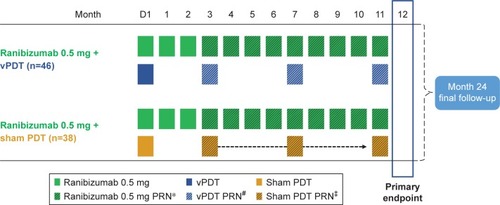
Table 1 Patient demographics, baseline disease, and ocular characteristics (randomized set)
Figure 2 Randomization and patient disposition in the Japanese cohort.
Abbreviation: vPDT, verteporfin photodynamic therapy.
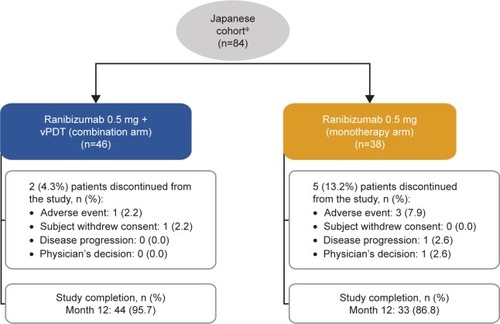
Figure 3 Assessment of BCVA outcomes over the 12-month study period.
Abbreviations: BCVA, best-corrected visual acuity; FAS, full analysis set; vPDT, verteporfin photodynamic therapy; LOCF, last observation carried forward; n, number of patients.
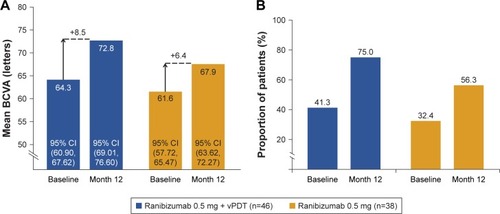
Figure 4 Proportion of participants with complete polyp regression by study visits up to Month 12 (FAS).
Abbreviations: FAS, full analysis set; ICGA, indocyanine green angiography; vPDT, verteporfin photodynamic therapy.
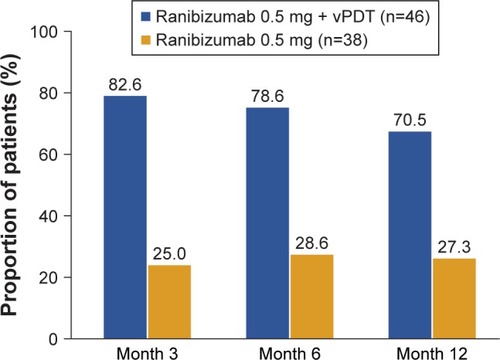
Figure 5 Mean change in CSFT and CCT, and proportion of participants with disease activity.
Abbreviations: CSFT, central subfield thickness; CCT, central choroidal thickness; FAS, full analysis set; M, month; vPDT, verteporfin photodynamic therapy.
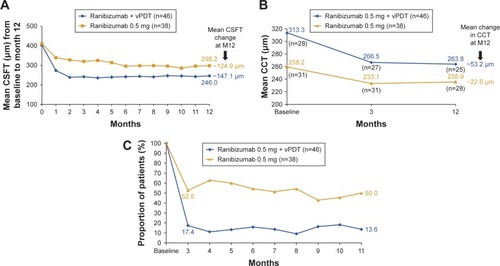
Table 2 Number of ranibizumab injections and vPDT/sham PDT treatments administered prior to month 12 in the Japanese cohort (safety set)
Table 3 Ocular AEs regardless of study drug relation ship up to month 12 (safety set)
