Figures & data
Figure 1 Patient disposition stratified by indication, history of DEX use, and number of on-study DEX injections.
Abbreviations: ATP, according to protocol; DEX, dexamethasone intravitreal implant; NIPSU, noninfectious posterior segment uveitis; RVO, retinal vein occlusion.
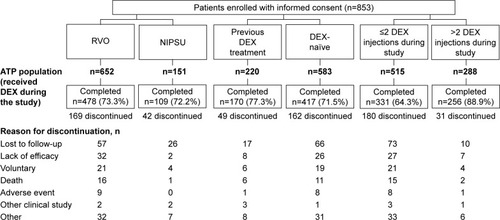
Table 1 Baseline patient characteristics (ATP population)
Table 2 Baseline characteristics of treated eyes (ATP population)
Table 3 Medical and ocular histories of patients at baseline (ATP population)
Table 4 Nonocular serious adverse events classified by primary system organ class in all patients and stratifications (ATP population)
Table 5 Ocular serious adverse events in all treated eyes and stratifications (ATP population)
Table 6 Adverse events of special interest in all treated eyes and stratifications (ATP population)
Table 7 Ocular procedures performed during the study in two or more treated eyes (ATP population)
Table S1 Incidence rates of ocular serious adverse events per person-year in all treated eyes and in treated eyes stratified by indication (ATP population)
Table S2 Incidence rates of adverse events of special interest per person-year in all treated eyes and in treated eyes stratified by indication (ATP population)
