Figures & data
Figure 1 Study design.
Abbreviations: AC, allergic conjunctivitis; CAC, conjunctival allergen challenge.
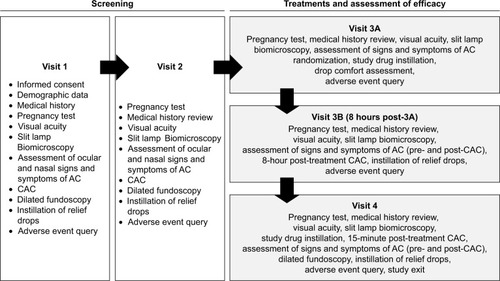
Table 1 Subject disposition
Table 2 Demographics
Table 3 Change in ocular itching and conjunctival redness
Figure 2 Ocular itching.
Abbreviations: AC, allergic conjunctivitis; CAC, conjunctival allergen challenge; PT, post-treatment.
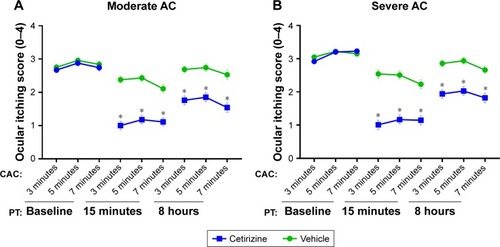
Table 4 Change in secondary ocular efficacy endpoints post-treatment, post-CAC, all time points
Figure 3 Eyelid swelling.
Abbreviations: AC, allergic conjunctivitis; PT, post-treatment; CAC, conjunctival allergen challenge.
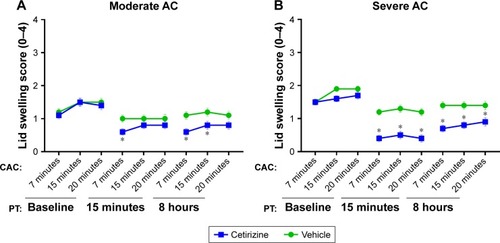
Table 5 Change in nasal symptoms post-treatment, post-CAC, all time points
Table 6 Proportion of subjects with nasal symptoms
Table 7 Drop comfort assessments
