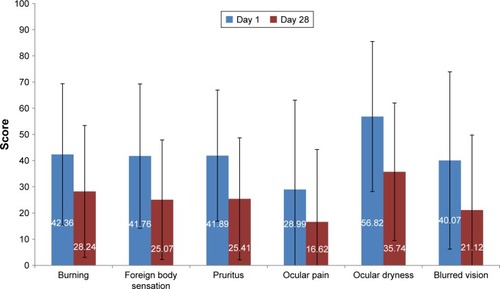
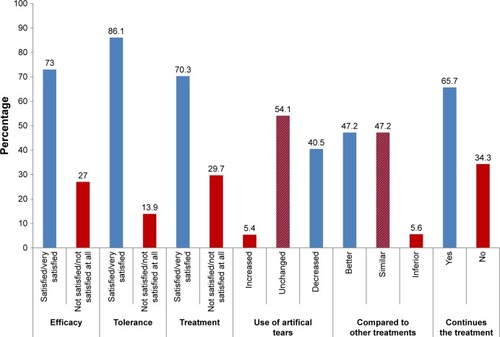
Figures & data
Table 1 Demographic data and clinical assessments at day 1
Figure 1 Oxford score at days 1 and 28.
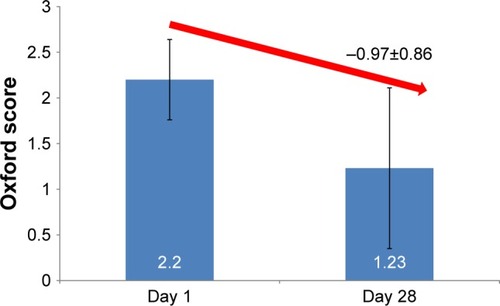
Figure 2 Tear break-up time, Schirmer score, intraocular pressure and visual acuity at days 1 and 28.
Abbreviations: IOP, intraocular pressure; TBUT, tear break-up time.
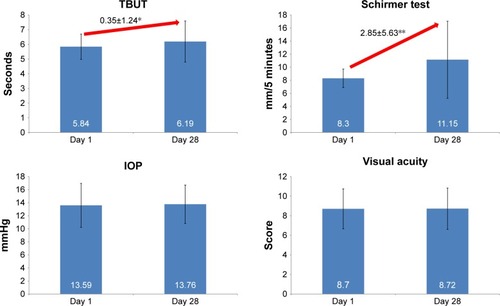
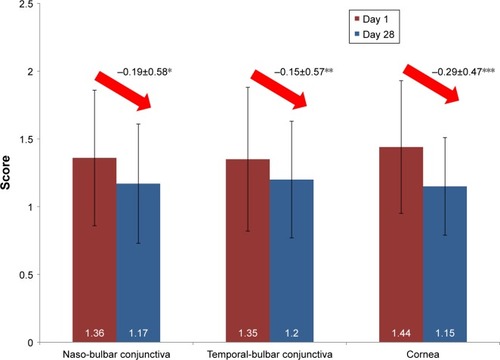
Figure 3 Ocular surface damage (mean scores) at days 1 and 28.