Figures & data
Figure 1 Study design. *One drop instilled at 09:00±30 min and 21:00±30 min. #One drop instilled at 09:00±30 min or 21:00±30 min. For the evening dose, patients waited for 15 min after instillation of TTFC, and then instilled the masked drug. **Study TTFC was administered at 21:00±30 min if the patient was dosed with TTFC in the evenings prior to Screening, or at 09:00±30 min on the following day if the patient was dosed with TTFC in the mornings prior to Screening.
Abbreviations: BBFC, brinzolamide 1%/brimonidine 0.2% fixed-dose combination; BID, twice daily, E1, eligibility visit 1, E2, eligibility visit 2; QD, once daily; TTFC, travoprost 0.004%/timolol 0.5% fixed-dose combination.
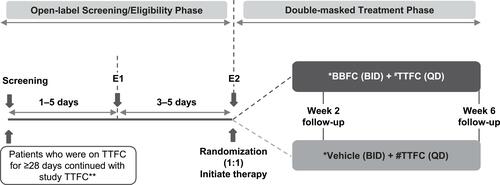
Table 1 Demographics And Baseline Characteristics (Full Analysis Set)
Figure 2 Patient disposition.
Abbreviations: AE, adverse event; BBFC, brinzolamide 1%/brimonidine 0.2% fixed-dose combination; N, total number of patients; n, number of patients; TTFC, travoprost 0.004%/timolol 0.5% fixed-dose combination.
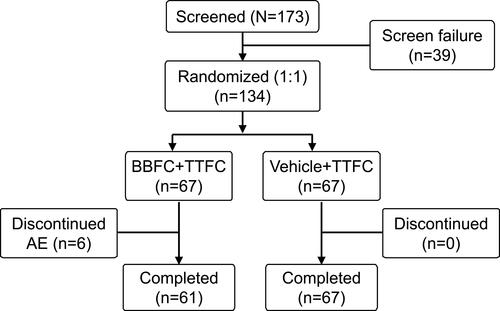
Figure 3 Mean change in diurnal intraocular pressure from baseline at Week 6 (Full analysis set). *BL IOP expressed as mean (SD) and defined as the average of 09:00 and 11:00 values.
Abbreviations: BBFC, brinzolamide 1%/brimonidine 0.2% fixed-dose combination; BL, baseline; CI, confidence interval; FAS, full analysis set; IOP, intraocular pressure; LS, least square; n, number of patients; SE, standard error; TTFC, travoprost 0.004%/timolol 0.5% fixed-dose combination.
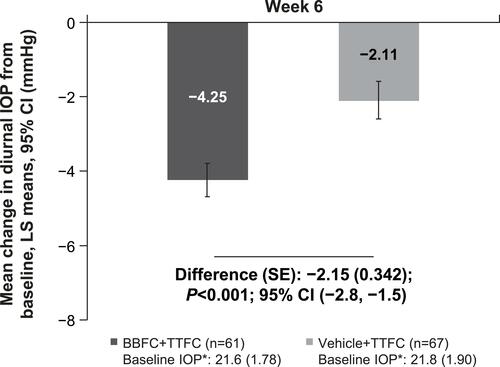
Figure 4 Mean diurnal intraocular pressure at week 6 (Full analysis set). *BL IOP expressed as mean (SD) and defined as the average of 09:00 and 11:00 values.
Abbreviations: BBFC, brinzolamide 1%/brimonidine 0.2% fixed-dose combination; CI, confidence interval; FAS, full analysis set; IOP, intraocular pressure; LS, least squares; n, number of patients; SD, standard deviation; SE, standard error; TTFC, travoprost 0.004%/timolol 0.5% fixed-dose combination.
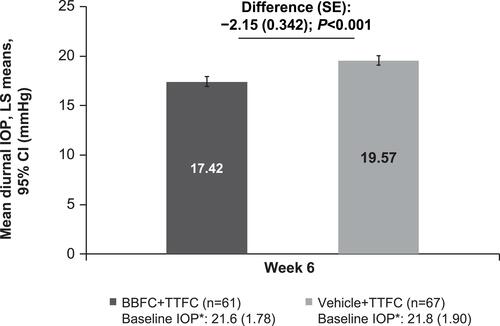
Table 2 Adverse Events (Safety Set)
