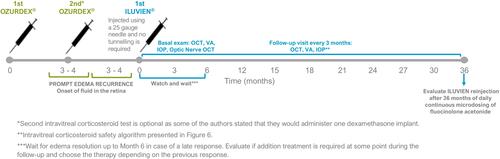
Figures & data
Table 1 Comparison of Available Intravitreal Corticosteroid Agents
Figure 1 FA levels in human aqueous humor in subjects receiving one ILUVIEN® implant (FAMOUS Study).
Notes: Data from Campochiaro et al.Citation12 FAc, 0.2 μg/day fluocinolone acetonide (ILUVIEN).
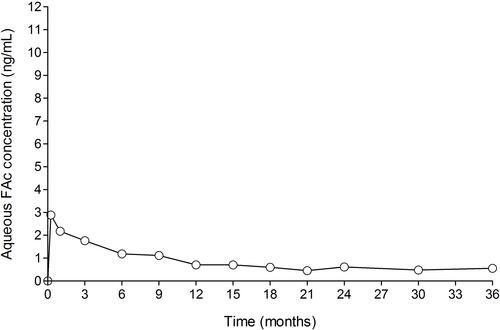
Table 2 Key Clinical Trial and Real-World Studies Demonstrating Effectiveness of 0.19 Mg Fluocinolone Acetonide Implant
Figure 2 Percentage of subjects with ≥15 letter improvement from baseline in best corrected visual acuity (BCVA) at 24 and 36 months in patients with DME treated with 0.2 μg/day FAc implant versus sham injection.
Notes: Data from Campochiaro et al.Citation7, and ILUVIEN® Spanish Summary of Product Characteristics.Citation70 *P = 0.002 FAc vs sham at Month 24; **P < 0.018 FAc vs sham at Month 36; ***P < 0.001 FAc vs sham for both at Month 24 and 36. Full study population, N= 376 (FAc), N = 185 (sham); Chronic DME subgroup, N = 207 (FAc), N = 111 (sham).
Abbreviation: DME, diabetic macular edema; FAc, 0.2 μg fluocinolone acetonide (ILUVIEN).
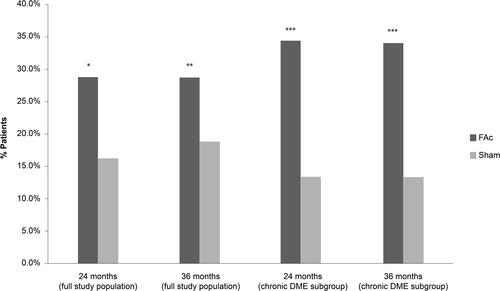
Figure 3 Mean gains in best corrected visual acuity (BVCA) from baseline at 24 and 36 months in patients with chronic DME treated with 0.2 μg/day FAc implant.
Notes: *P = 0.35 vs sham at Month 24; **P = 0.04 vs sham at Month 36.; FAc, 0.2 μg fluocinolone acetonide.
Abbreviations: DME, diabetic macular edema; FAc, 0.2 μg fluocinolone acetonide (ILUVIEN®).
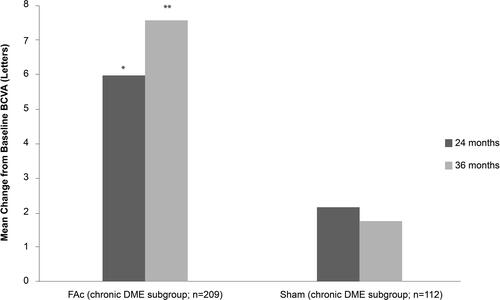
Figure 4 Mean central foveal thickness (CFT) change in patients with chronic DME treated with 0.2 μg/day FAc implant.
Notes: Data from Campochiaro et al.Citation7, and ILUVIEN® Spanish Summary of Product Characteristics.Citation70 *P = 0.005 vs sham at Month 24.
Abbreviation: DME, diabetic macular edema; FAc, 0.2 μg fluocinolone acetonide (ILUVIEN).
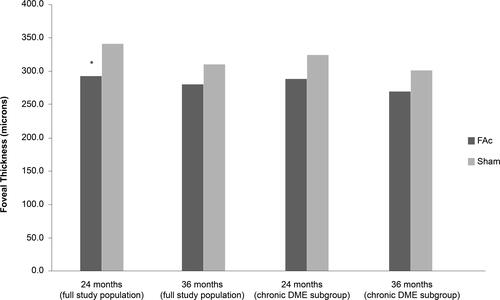
Figure 6 Algorithm for the management of IOP elevation by retinal specialists: pre- (top panels) and post-corticosteroid injection (bottom panels).
Note: Adapted from Goni et al.Citation93
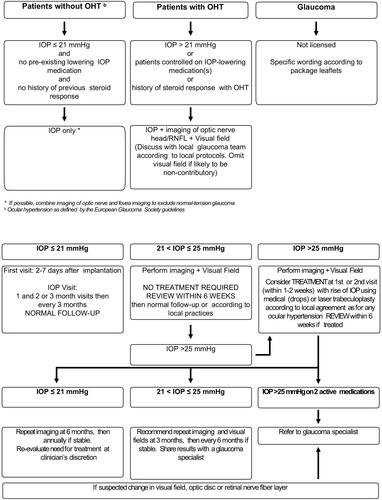
Table 3 General Pre- and Post-Injection Action Protocol for FAc Implant Treatment