Figures & data
Figure 1 Study design.
Abbreviations: EOS, end of study; QID, four times daily; RFO-Ad, Refresh Optive Advance/Optive Plus; SYSB, Systane Balance; TFBUT, tear film break-up time.
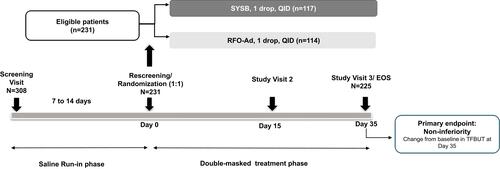
Table 1 Demographic Characteristics of Patients by Treatment Groups–Randomized Set
Figure 2 The change from baseline in TFBUT at Day 35 (test for non-inferiority, primary endpoint), by treatment group-PPS population.
Notes: Non-inferiority was deemed established if the lower limit of the 95% CI (equivalent to the 1-sided 97.5% CI) for the adjusted estimate of the difference (SYSB−RFO-Ad) was above −1.0 s.
Abbreviations: CI, confidence interval; LS, least squares; PPS, per-protocol set; RFO-Ad, Refresh Optive Advance/Optive Plus; SE, standard error; SYSB, Systane Balance; TFBUT, tear film break-up time.
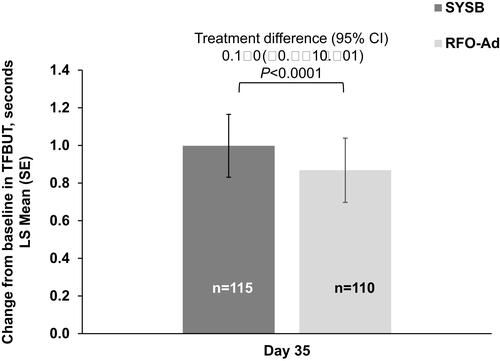
Table 2 Summary of TFBUT, Global Ocular Discomfort VAS Score, and IDEEL Treatment-Satisfaction Scores (Effectiveness and Inconvenience) by Treatment Group at Each Study Visit- FAS Population
Figure 3 Change from baseline in IDEEL treatment satisfaction scores (treatment effectiveness and treatment inconvenience scores) at Day 35, by treatment group-FAS population.
Note: P value<0.05 for a positive treatment difference (SYSB – RFO-Ad) would have favored SYSB.
Abbreviation: CI, confidence interval; FAS, full analysis set; IDEEL, impact of dry eye on everyday life; LS, least squares; RFO-Ad, Refresh Optive Advance/Optive Plus; SE, standard error; SYSB, Systane Balance.
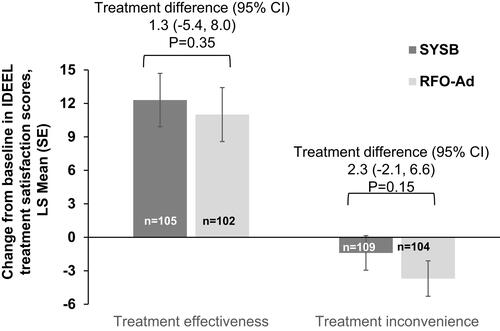
Table 3 Proportion of Patients with Common Adverse Events (≥1.0% Incidence) by Preferred Term-Safety Analysis Set
