Figures & data
Figure 1 Formation of the micellar formulation. The micellar formulation consists of latanoprost, an NIS, castor oil, a buffering agent, and a safer sorbate-based preservative system. NIS functions as the primary solubilizer, while castor oil has a dual function of a demulcent and stabilizer.
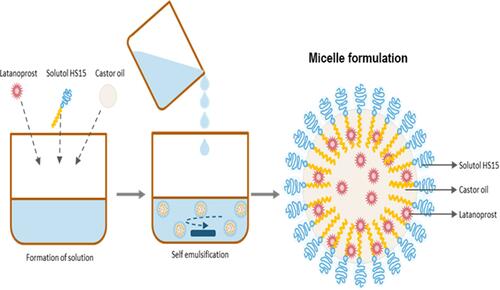
Figure 2 Optimization of the concentrations of (A) castor oil and (B) total lipid concentration. (A) The optimum concentration is located at the intersection of the zone of clarity and the zone producing the micellar formulation. (B) The optical transmission of total lipid concentrations 0.4% to 1.6% were measured against the total lipid concentration. The intersection of these 2 lines identifies the optimal total lipid concentration for the micellar formulation.
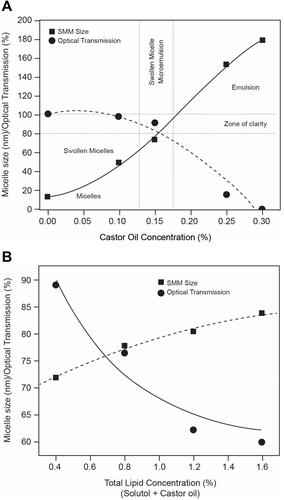
Table 1 Characteristics of Surfactant-Oil Pairs
Table 2 Interbatch Particle Size Distribution
Table 3 Physicochemical Characteristics of the Micellar Formulation Across the 6 Batches
Table 4 Average Potency of Latanoprost in Stability Study
Table 5 In vitro Dilution Study
Table 6 Cytotoxicity of Positive and Negative Controls and Micellar Formulation with Latanoprost 0.005% and Latanoprost 0.005% with BAK in Neutral Red Assay
