Figures & data
Figure 2 Loteprednol etabonate molecular structure: loteprednol etabonate is synthesized through structural modifications of a prednisolone-related compound.
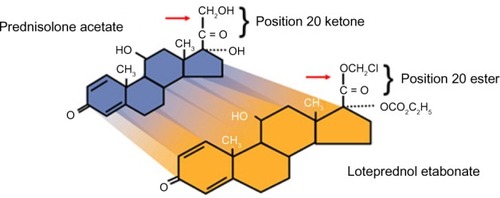
Figure 3 Change in intraocular pressure after treatment with Lotemax® ointment or vehicle.
Abbreviation: Lotemax, Ioteprednol etabonate.
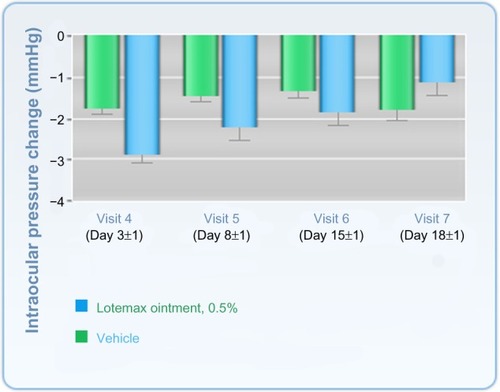
Table 1 Published clinical studies on Lotemax® and penetrating keratoplasty
Table 2 Comparative studies on efficacy and safety of Lotemax® 0.5% in resolution of postoperative inflammation