Figures & data
Figure 1 Disposition of Korean patients with nAMD. The safety set comprised patients in the enrolled set who were treated with at least one dose of ranibizumab during the study or prior to study initiation and had at least one safety assessment post-initial treatment. Patients with a baseline visit on or before March 2015 were included.
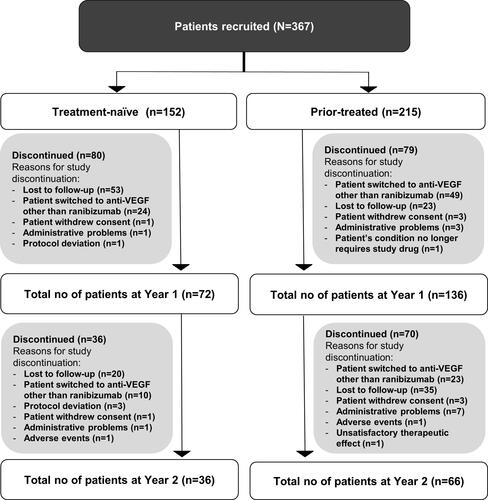
Table 1. Baseline Demographic, Patient Characteristics and Treatment Pattern at Year 1: Safety Set and Primary Treated Eye Set
Figure 2 Mean change from baseline in visual acuity (ETDRS letter score): primary treated eye set. Primary treated eye set included all primary treated eyes in patients included in the safety set. Mean ± SE was presented. The study entry date was defined as baseline date if the primary-treated eye has been pre-treated with ranibizumab. If the eyes were not pre-treated with ranibizumab, the date of the first on-study ranibizumab injection was considered as the baseline date. Statistical analyses were performed using two-sample Student’s t-tests to compare VA between baseline and specific time point, **P<0.01, ***P<0.001.
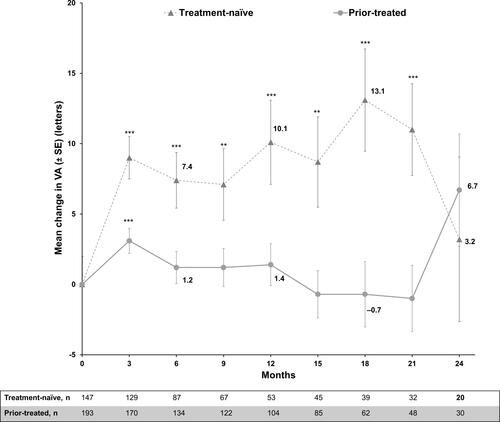
Figure 3 Mean change in VA from baseline to Month 12 in primary treated eye set by (A) pre-treatment status; (B) baseline VA. Observed data set for VA change in primary treated eye set. Primary treated eye set included all primary treated eyes in patients included in the safety set. Statistical analyses were performed using two-sample Student’s t-tests to compare VA between baseline and Year 1, ***P<0.001.
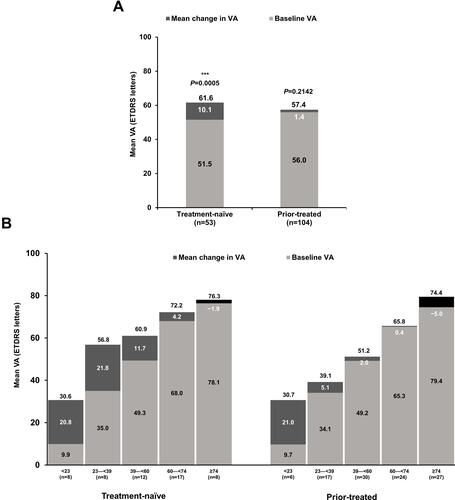
Figure 4 Mean change in VA from baseline to Month 12 in primary treated eye set by (A) loading dose status (B) injection number. Observed data set for VA change in primary treated eye set. Primary treated eye set included all primary treated eyes in patients included in the safety set. Loading dose was defined as receiving at least 3 ranibizumab injection within 120 days from baseline date.
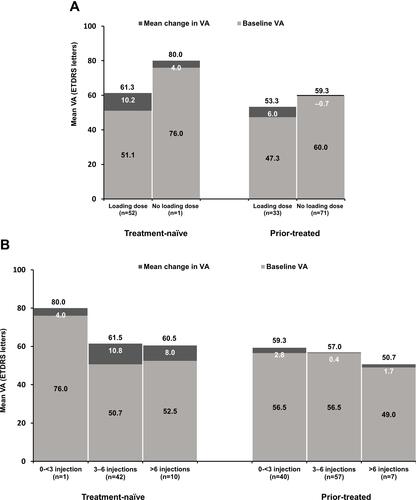
Figure 5 Mean change in VA from baseline to Month 12 by PCV status: primary treated eye set. Observed data set for VA change in primary treated eye set. Primary treated eye set included all primary treated eyes in patients included in the safety set. Statistical analyses were performed using two-sample Student’s t-tests to compare the mean change in VA between patients with/without PCV, *P < 0.05.
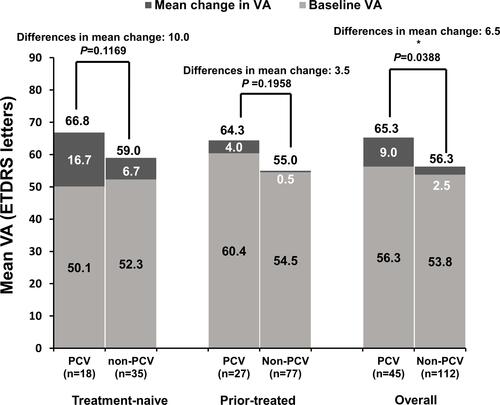
Table 2. Association of Visual Acuity Change with Baseline Characteristics and Treatment Pattern in Korean Patients with nAMD: Safety Set and Primary Treated Eye Set
Figure 6 Frequency of visit and injections over 12 months in Korean patients with nAMD and VA assessed at Month 12 in primary-treated eye and safety sets (A) treatment-naïve patients; (B) prior-treated patients. The safety set comprised patients in the enrolled set who were treated with at least one dose of ranibizumab during the study or prior to study initiation and had at least one safety assessment post-initial treatment. Primary treated eye set included all primary treated eyes in patients included in the safety set.
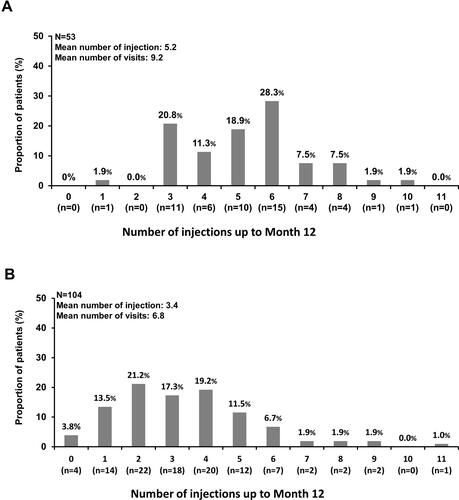
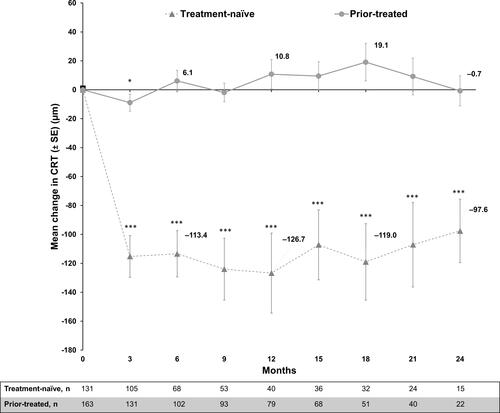
Figure 7 Mean change from baseline in CRT (µm): Primary treated eye set. Primary treated eye set included all primary treated eyes in patients included in the safety set. Mean ± SE was presented. The study entry date was defined as baseline date if the primary-treated eye has been pre-treated with ranibizumab. If the eyes were not pre-treated with ranibizumab, the date of the first on-study ranibizumab injection was considered as the baseline date. Statistical analyses were performed using two-sample Student’s t-tests to compare CRT between baseline and specific time point, *P<0.05, ***P<0.001.