Figures & data
Table 1 Patient demographics and baseline characteristics
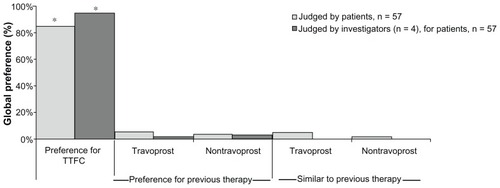
Figure 1 Mean IOP across visits by patient cohort.
**P < 0.0001, baseline versus week 4 or week 12, as measured by ANOVA.
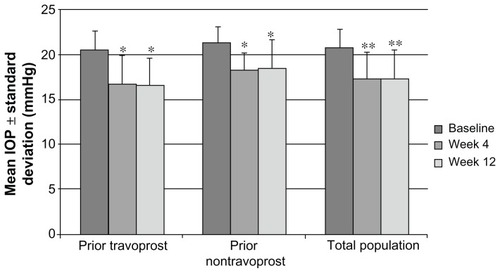
Figure 2 Percentage of patients reaching target IOP across visits by patient cohort.
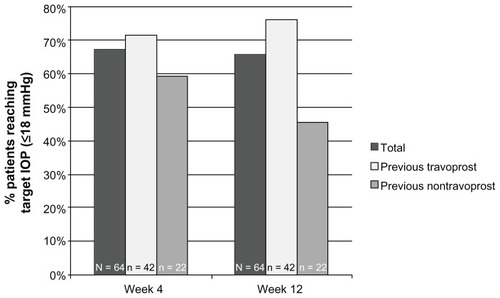
Figure 3 Percentage of patients achieving ≥2 mmHg reductions in IOP across visits by patient cohort.
Table 2 Adverse events of all enrolled patients (N = 65)
Table 3 Survey results, with ocular symptoms at week 12 sorted from lowest to highest incidence