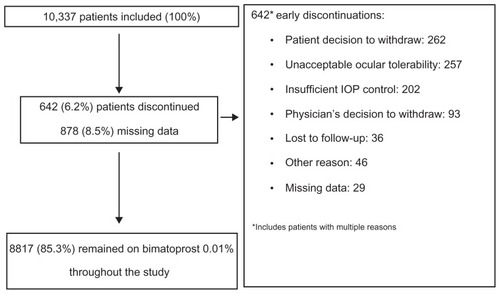
Figures & data
Table 1 Prior medications taken by >2% of patients, among those whose prior therapy was documented (n = 8441)Table Footnotea
Figure 2 Percentage reduction from baseline in mean IOP in all patients and in those receiving prior monotherapy (complete data) at 10–14 weeks following initiation of bimatoprost treatment.
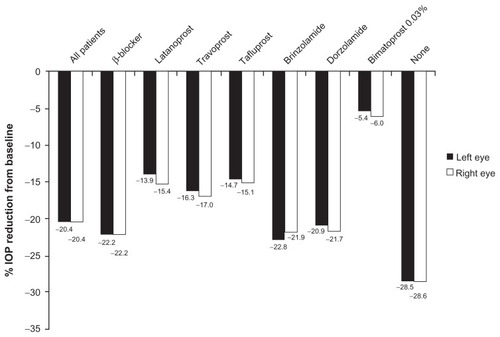
Table 3 Baseline, final, and reduction from baseline in mean IOP (mmHg) in all patients and in those receiving prior monotherapy (complete data) at 10–14 weeks following initiation of bimatoprost treatment
Table 2 Physician’s evaluation of achievement of target IOP in all patients, patients on prior β-blocker or prostaglandin analog monotherapy, and in patients on no prior therapy
Table 4 Adverse events occurring in ≥0.1% of the total population (n = 10,337) classified according to MedDRA 13.1 preferred term