Figures & data

Table 1 Participant Disposition, Demographics, Treatment Exposure, and Compliance, by Treatment Group
Figure 1 Mean ± standard deviation (A) intraocular pressure (IOP), (B) pupil diameter, and (C) Snellen visual acuity (VA) at selected time points. Results shown are for OD. Data from N=568 participants enrolled in four oxymetazoline 0.1% clinical trials ranging in duration from 14 to 84 days, except IOP, which presents data from N=538 participants enrolled in three phase 3 trials ranging in duration from 42 to 84 days. Includes data from participants from two phase 3 efficacy studies 42 days in duration with data previously reported by Slonim et al 2020.Citation41 In the phase 1/2a study, pupil diameter and Snellen VA were evaluated at screening and on treatment days 1 and 14, and IOP was evaluated at screening and on treatment days 7 and 14. As previously reported, in the phase 3 efficacy trials, pupil diameter and Snellen VA were evaluated at all study visits, and IOP was evaluated at screening and on treatment day 42. In the phase 3 safety trial, pupil diameter and Snellen VA were evaluated at all study visits, and IOP was evaluated at baseline/screening and on treatment days 42 and 84.
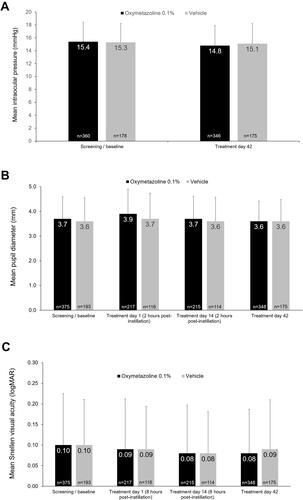
Table 2 Summary of Treatment-Emergent Adverse Events (TEAEs)
Table 3 Summary of Serious Treatment-Emergent Adverse Events (TEAEs)
Table 4 Most Common Treatment-Emergent Adverse Events (TEAEs; Occurring in >1% of Patients in Either Treatment Arm)
Table 5 Relationship to Treatment and Severity of Treatment-Emergent Adverse Events (TEAEs)
