Figures & data
Table 1 Demographic Characteristics of the Study Population
Table 2 Differences in the Pre- and Post-Intravitreal Brolucizumab Injection Variables
Figure 1 Representative case of a treatment-naïve nAMD showing complete resolution of subretinal fluid along with significant reduction in the subretinal hyperreflective material (SHRM) at weeks 4 (B), 8 (C), and 12 (D) as compared to the baseline (A) after a single dose of intravitreal brolucizumab injection.
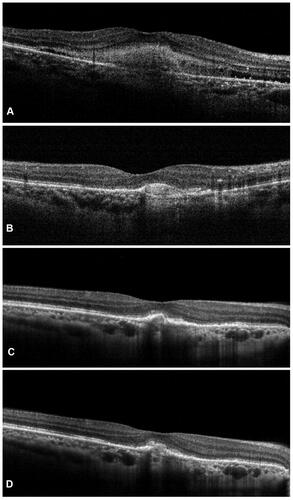
Figure 2 Representative case of nAMD who had previously received multiple anti-vascular endothelial growth factor (anti-VEGF) injections with suboptimal outcomes. After switching to intravitreal injection (IVI) brolucizumab, the patient demonstrated complete resolution of the subretinal and intraretinal fluid at weeks 4 (B) and 8 (C) as compared to the baseline (A). Early recurrence was noted at 12 weeks (D) for which the patient underwent second dose of IVI brolucizumab.
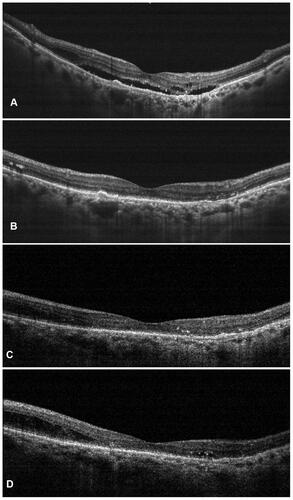
Table 3 List of Adverse Events in Patients Receiving Intravitreal Brolucizumab Injections
