Figures & data
Table 1 Demographic and Baseline Clinical Characteristics of SONATA and RWE Chart Review Studies
Figure 1 Proportion of patients who did not use artificial tears in the lifitegrast and placebo groups of the SONATA study (safety population).
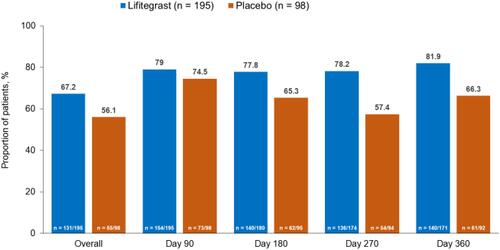
Figure 2 Proportion of patients with DED-related treatments during the pre- and post-index period in the RWE chart review. *Only medication reported in the medical charts are reported as a post-index combination therapy with lifitegrast.
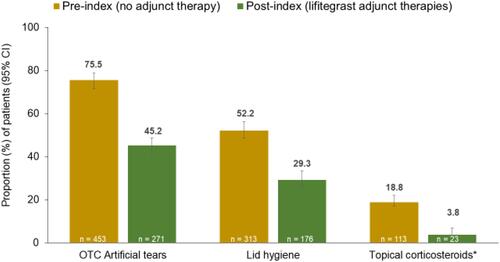
Table 2 DED Symptoms Reported During the Preindex and Postindex Periods in the RWE Chart Review Study
