Figures & data
Table 1 Demographics and Baseline Characteristics of Master Cohort
Table 2 Demographics and Baseline Characteristics of Patients with a Diagnosis of PCOa After Cataract Surgery
Figure 1 Incidence of Nd:YAG due to PCO (A) by type and (B) by brand of IOL. Nd:YAG laser capsulotomy due to PCO was defined as the presence of an Nd:YAG procedure code (CPT 66821) with an accompanying PCO diagnosis code (ICD-10 code H26.491 or H26.492 or H26.493 or H26.499) for the same eye on the date of the Nd:YAG procedure or between the Nd:YAG procedure and the cataract procedure.
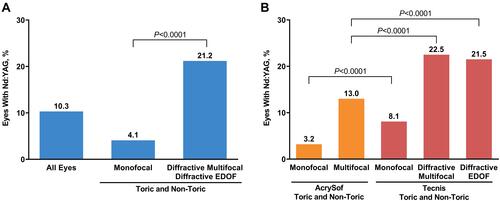
Figure 2 Risk of Nd:YAG due to PCO by IOL type or brand, baseline characteristics, and region. Nd:YAG laser capsulotomy due to PCO was defined as the presence of an Nd:YAG procedure code (CPT 66821) with an accompanying PCO diagnosis code (ICD-10 code H26.491 or H26.492 or H26.493 or H26.499) for the same eye on the date of the Nd:YAG procedure or between the Nd:YAG procedure and the cataract procedure.
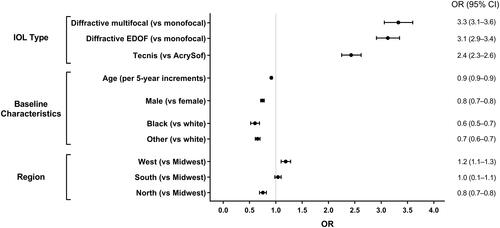
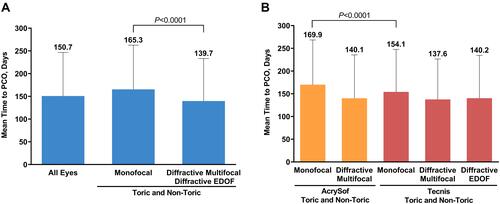
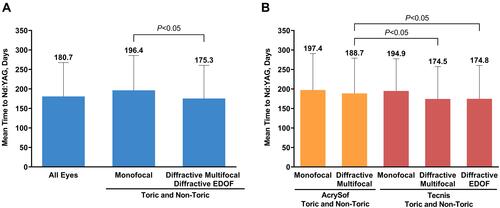
Figure 3 Time to PCO diagnosis (A) by type and (B) by brand. PCO was defined as the presence of a PCO diagnosis code (ICD-10 code H26.491 or H26.492 or H26.493 or H26.499) within 365 days of the cataract procedure.