Figures & data
Table 1 Summary of Baseline Patient Characteristics for the T2345 and BPL Treatment Groups (ITT Population)
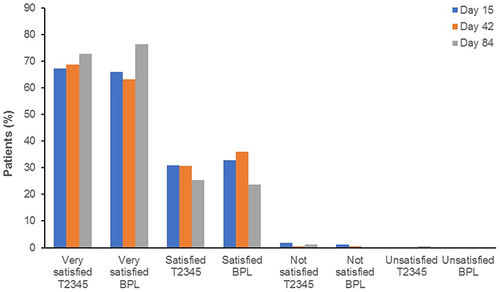
Figure 1 Patient disposition (a), and study design (b), specifying the timings of screening, washout periods, and assessment schedule timings. *One patient was initially assigned a specific randomization number, but the study medication for this number (BPL) was unusable because it had frozen on the delivery dock. In accordance with the protocol, the patient was assigned the next randomization number, which specified T2345 administration. This patient was analyzed with the T2345 group for the safety population (actual treatment) and with the BPL group for the ITT population. The patient was excluded from the PP population. **Patient had ≥1 discontinuing reason. AE, adverse event; BAK, benzalkonium chloride; BPL, BAK-preserved latanoprost 0.005%; ITT, intent-to-treat; LTFU, lost to follow-up; PP, per protocol; T2345, preservative-free single-dose latanoprost 0.005%.
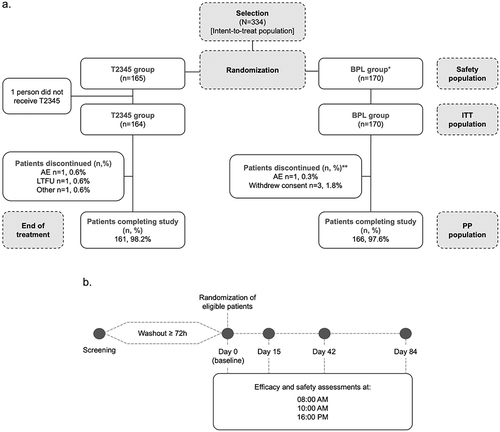
Figure 2 Mean IOP values in the study eye in the T2345 and BPL-treated groups (a) and difference in IOP (mm Hg) change from baseline levels between each group at each time point (intent-to-treat population; (b), and in the contralateral eye (c and d).
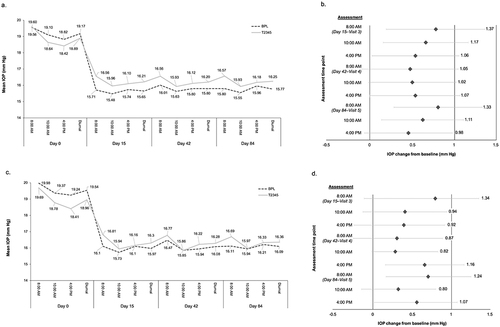
Table 2 Statistical Analyses of Noninferiority Criteria
Figure 3 Proportion of patients with IOP <18 mm Hg in the study eye (per-protocol population).
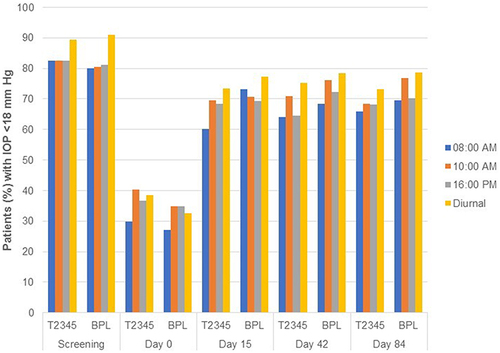
Table 3 Number (%) of Patients with Most Common Ocular Treatment-Emergent Adverse Events with Relationship to Study Medicine by Preferred Term (Safety Population)