Figures & data
Figure 1 ADNIV disease stages. (A) Stage III ADNIV shows epiretinal membrane and tractional edema. (B) Stage IV shows tractional retinal detachment.
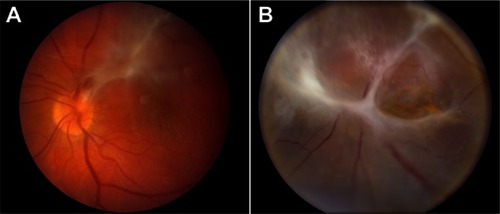
Figure 2 ADNIV pedigree and Clinical Images. (A) Patient A. CAPN5 genotype and family pedigree; representative OCT demonstrating a thick ERM and CME in the macula; and echography reveals a peripheral TRD in Stage IV disease; the fundus drawing shows inferior, anterior PVR membranes encapsulating the implant (Retisert™, Bausch & Lomb, Rochester, NY, USA). (B) Patient B. CAPN5 genotype and family pedigree; representative OCT showing a thick ERM and tractional elevation of the macula; echography suggests early phthisis; and the fundus drawing shows anterior neovascularization and inferior PVR membranes encapsulating the implant. In the pedigrees, arrows designate the patient with Stage IV disease and fibrotic encapsulation of the FA implant. Black symbols represent clinically affected subjects, and those that are labeled as Stage III have undergone FA implant without subsequent fibrotic encapsulation.
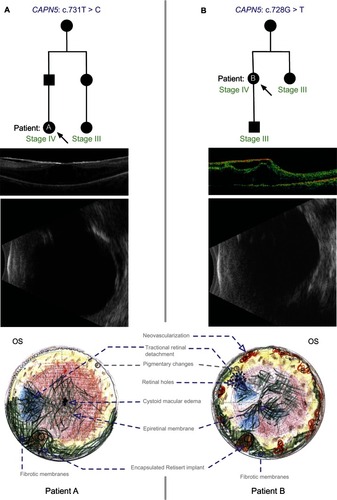
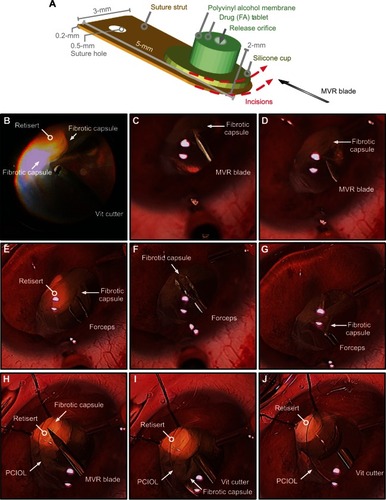
Figure 3 Surgical dissection in patient-A of fibrotic encapsulation of fluocinolone acetonide implant (Retisert™, Bausch & Lomb, Rochester, NY, USA). (A) Diagram detailing the dimensions of the fluocinolone acetonide implant. The dotted red lines indicate the placement and orientation of incisions created in the surrounding fibrotic capsule with a microvitreoretinal (MVR) blade. (B) The vitreous cutter was used to debulk any residual vitreous surrounding the fibrotic capsule. (C and D) The MVR blade was used to incise the fibrotic capsule, taking care to avoid contact with the delicate membrane of the implant. (E–G) Intraocular forceps were used to grasp the cut edge of the membrane and peel the membrane from the surface of the implant. (H) The MVR blade was used once more to ensure that all surrounding membranes were incised. (I, J) The vitreous cutter was used to trim and excise any residual capsule, revealing the drug capsule.