Figures & data
Figure 1 Participant flow chart.
Abbreviations: BBFC, brinzolamide/brimonidine fixed combination; IOP, intraocular pressure; ITT, intent-to-treat.
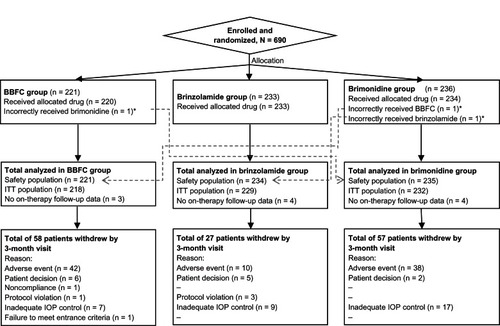
Table 1 Demographics and baseline characteristics
Table 2 Treatment-related adverse events (incidence ≥1%)
Table 3 Changes in ocular signs from baseline to any visit through month 6
Table 4 Changes in fundus parameters from baseline to any visit through month 6
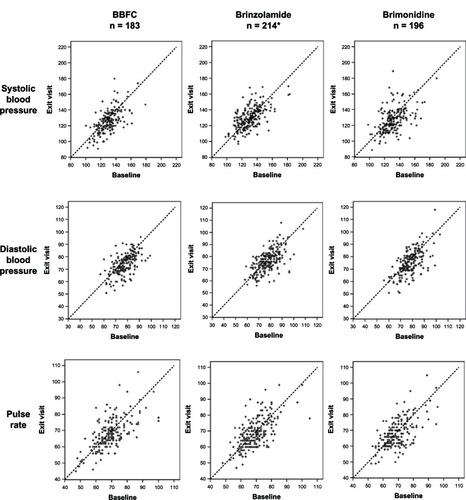
Figure 2 Distribution of systolic and diastolic blood pressure and pulse rate at 10 am: baseline visit versus six-month visit.
Abbreviation: BBFC, brinzolamide/brimonidine fixed combination.