Figures & data
Table 1 Patient demographics (full analysis set)
Figure 1 Evolution of cardinal signs of bacterial conjunctivitis.
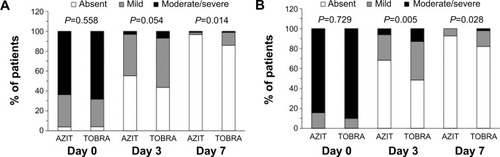
Figure 2 Global efficacy assessment by the investigator.
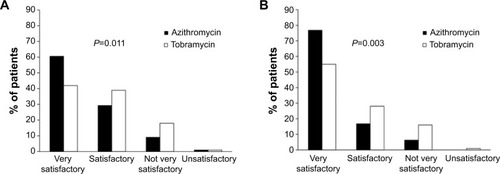
Figure 3 Number of days that an improvement was felt.
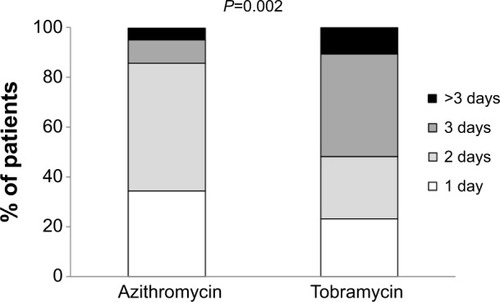
Figure 4 Ease of treatment and impact on daily life.
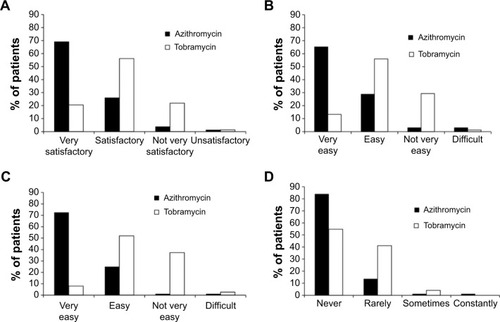
Table 2 Summary of questionnaire results among infants (aged less than 24 months)
