Figures & data
Figure 1 Study flow diagram.
Abbreviation: CAC, conjunctival allergen-challenge.
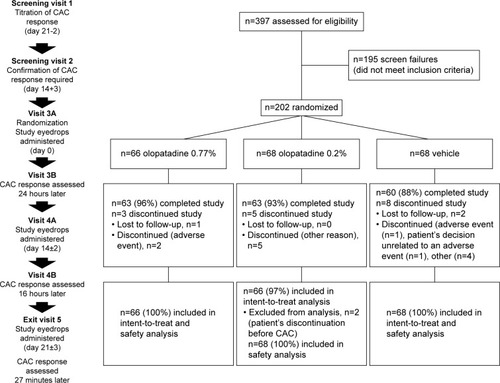
Table 1 Demographic and baseline characteristics (intent-to-treat population)Table Footnotea
Figure 2 Treatment differences in means after conjunctival allergen-challenge (CAC): primary end points (intent-to-treat population).
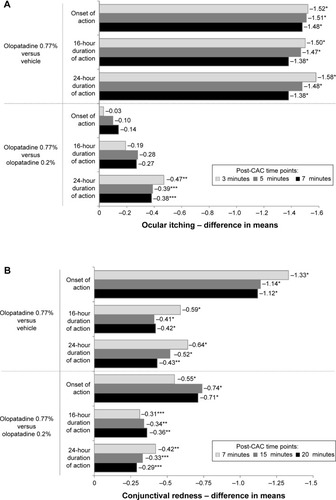
Figure 3 Treatment differences in means after conjunctival allergen-challenge (CAC): supportive end points (intent-to-treat population).
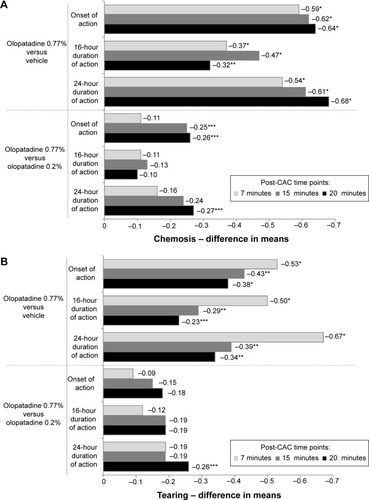
Table 2 Summary of treatment-emergent AEs (safety population)
