Figures & data
Figure 1 Many cancer cells are able to evade apoptosis through impairment of the mitochondrial apoptotic pathway, controlled by proapoptotic (eg, BAK, BAX, BIM) and prosurvival (eg, BCL2, BCL-XL) members of the BCL2 family.
Abbreviation: CLL, chronic lymphocytic leukemia.
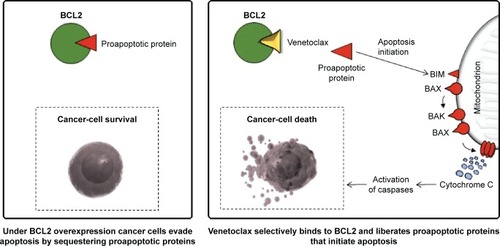
Figure 2 Durability of benefit with ongoing venetoclax therapy.
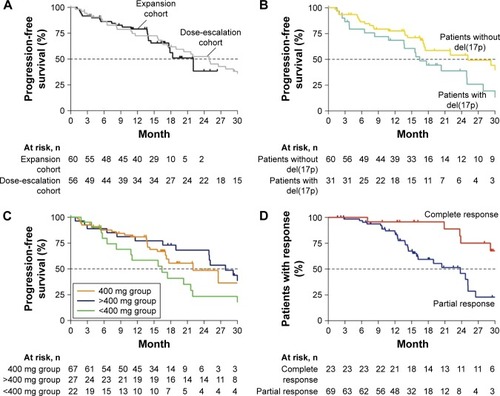
Figure 3 Response to venetoclax.
Abbreviations: CR, complete remission; CRi, CR with incomplete recovery of blood counts; nPR, nodular partial response.
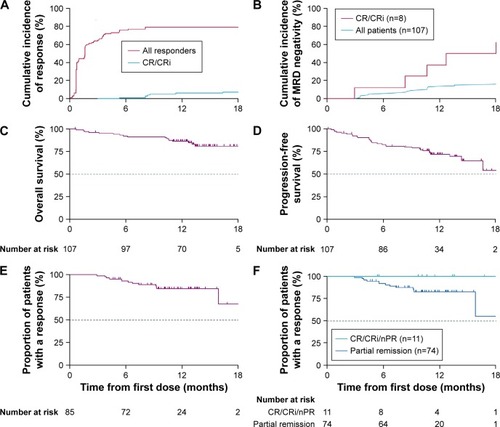
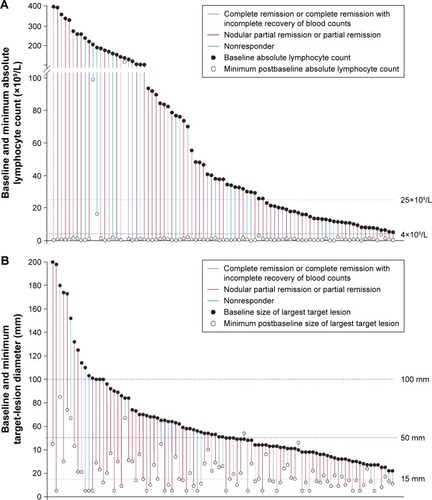
Figure 4 Venetoclax activity by compartment.