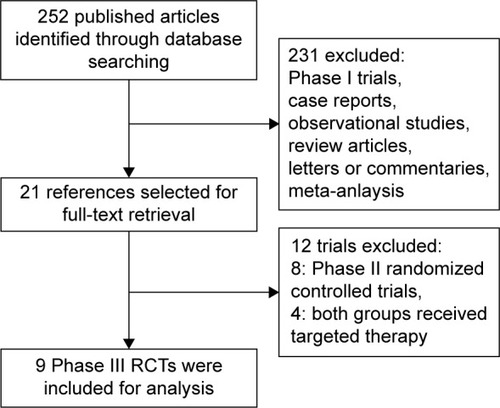
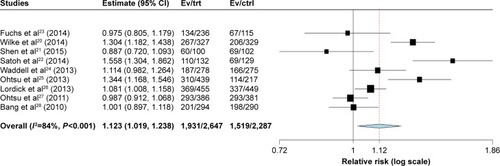
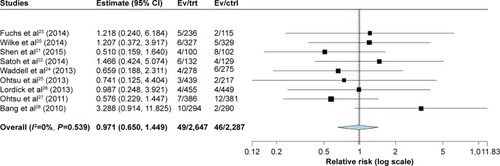
Figures & data
Table 1 Baseline characteristics of nine included trials
Table 2 FAEs by specific type