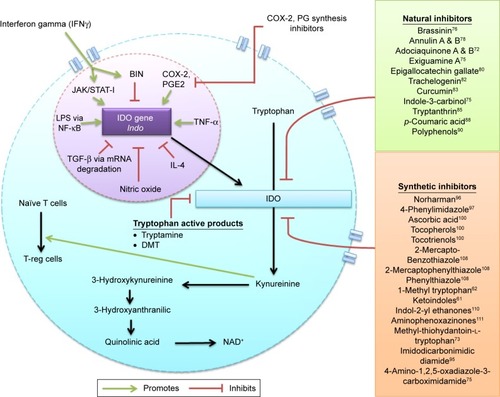
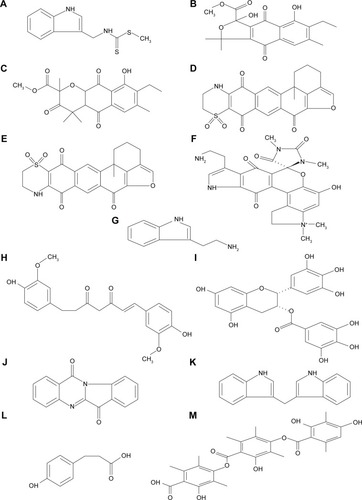
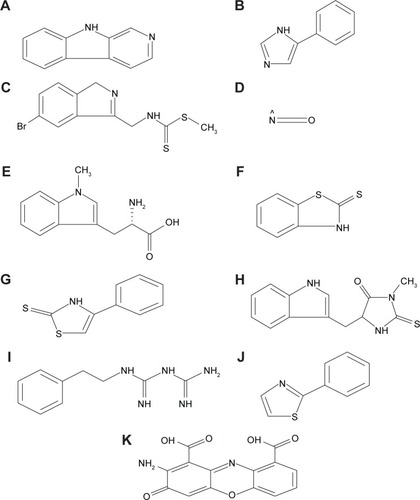
Figures & data
Table 1 Nanotechnological carrier systems used for cancer treatments
Table 2 US FDA approved nanomedicines used for cancer treatments
Table 3 Physicochemical properties of selected natural inhibitors
Table 4 Physicochemical properties of selected synthetic inhibitors