Figures & data
Table 1 Inclusion and exclusion criteria for study selection in this meta-analysis
Table 2 Baseline characteristics of included studies comparing nivolumab to other drugs
Table 3 The statistical methods used in this meta-analysis and their explanation
Figure 1 Literature search and selection of articles.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews.
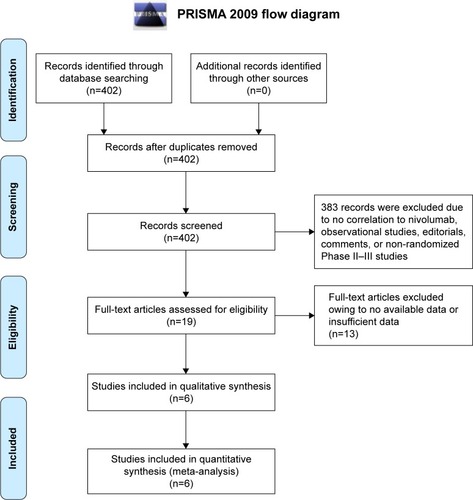
Table 4 Jadad quality assessment of the included studies
Figure 2 Labbe plots, sensitivity analysis plots and contour-enhanced funnel plots of the included studies focusing on the risk of selected neurotoxicity associated with the PD-1 inhibitor nivolumab.
Abbreviation: PD-1, programmed death-1.
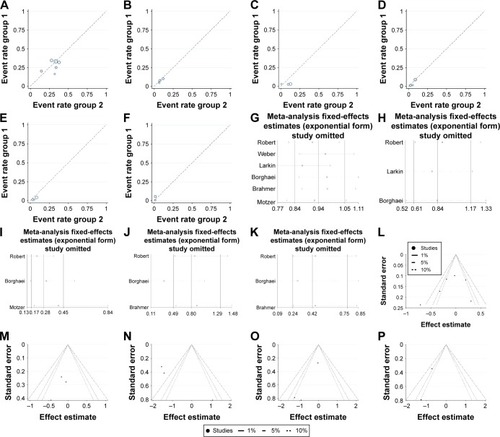
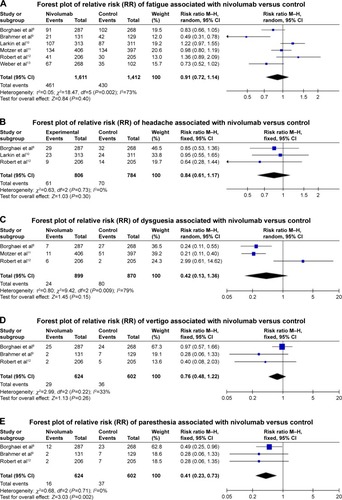
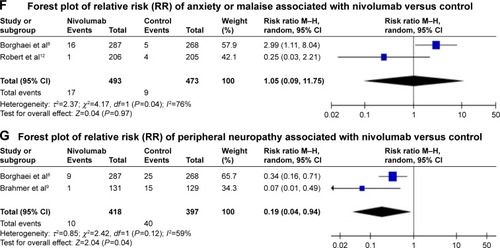
Figure 3 Forest plots (individual and pooled effects with 95% CI) regarding the risk of selected neurotoxicity of fatigue (A, random-effects model), headache (B, fixed-effects model), dysgeusia (C, random-effects model), vertigo (D, fixed-effects model), paresthesia (E, fixed-effects model), anxiety or malaise (F, random-effects model) and peripheral neuropathy (G, random-effects model) associated with nivolumab versus controls.