Figures & data
Figure 1 Consort diagram.
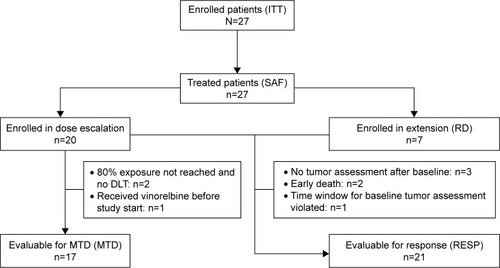
Table 1 Demographic data and disease characteristics
Table 2 Dose escalation – DLTs observed during the dose escalation phase of the trial including toxicities observed at the MTD
Figure 2 Vinorelbine blood concentrations on day 1 and day 21 (cycle 1) after daily dosing of oral vinorelbine.
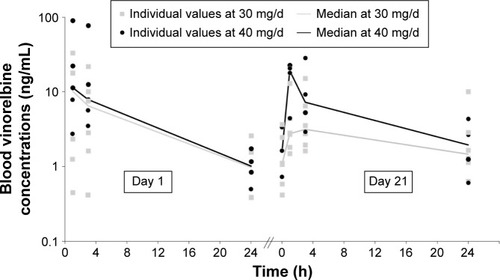
Table 3 AEs potentially related to vinorelbine occurring in at least 10% of the SAF and/or RD population
Table 4 Efficacy parameters
