Figures & data
Figure 1 Expression levels of TUG1 and ZEB2 in bladder cancer tissues (n=36) and corresponding nontumor tissues (n=36).
Abbreviations: mRNA, messenger RNA; N, adjacent normal tissues; T, tumor tissues; TUG1, taurine upregulated gene 1; ZEB2, zinc finger E-box binding homeobox 2.
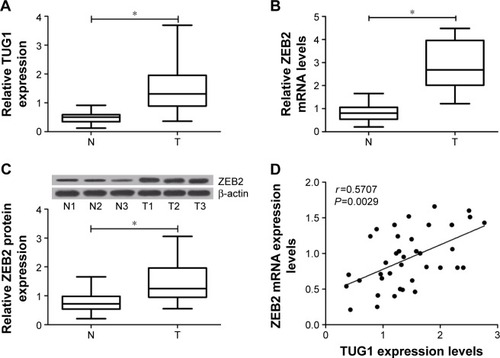
Figure 2 Knockdown of TUG1 suppressed bladder cancer cell proliferation and induced apoptosis in vitro.
Abbreviations: FITC, fluorescein isothiocyanate; NC, negative control; OD, optical density; PI, propidium iodide; si, small interfering; TUG1, taurine upregulated gene 1.
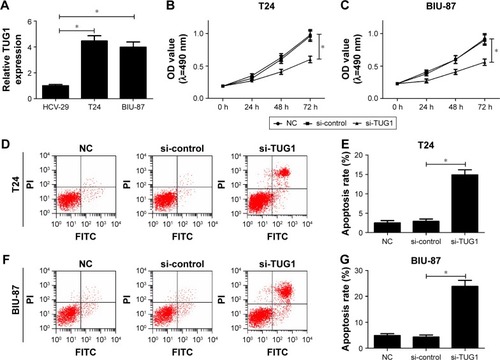
Figure 3 Knockdown of ZEB2 suppressed bladder cancer cell proliferation and induced apoptosis in vitro.
Abbreviations: mRNA, messenger RNA; NC, negative control; OD, optical density; si, small interfering; ZEB2, zinc finger E-box binding homeobox 2.
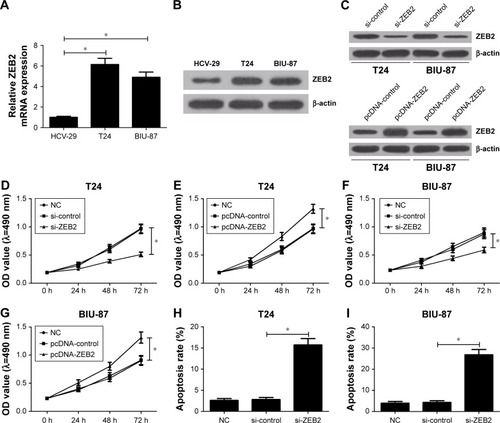
Figure 4 ZEB2 overexpression reversed effects of TUG1 knockdown on proliferation and apoptosis of bladder cancer cells.
Abbreviations: NC, negative control; OD, optical density; si, small interfering; TUG1, taurine upregulated gene 1; ZEB2, zinc finger E-box binding homeobox 2.
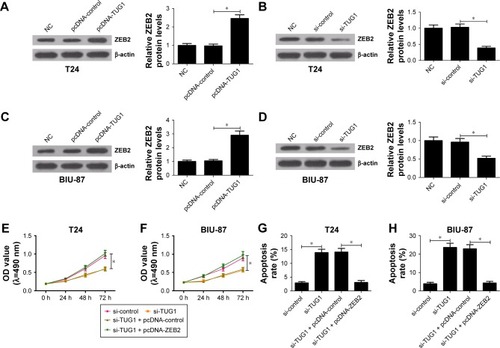
Figure 5 TUG1 targeted miR-142 to regulate ZEB2 expression.
Abbreviations: MUT, mutant; NC, negative control; qRT-PCR, quantitative real time polymerase chain reaction; si, small interfering; TUG1, taurine upregulated gene 1; ZEB2, zinc finger E-box binding homeobox 2; WT, wild type.
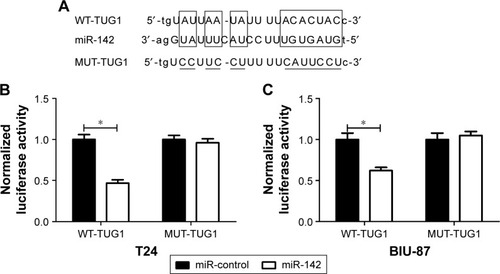
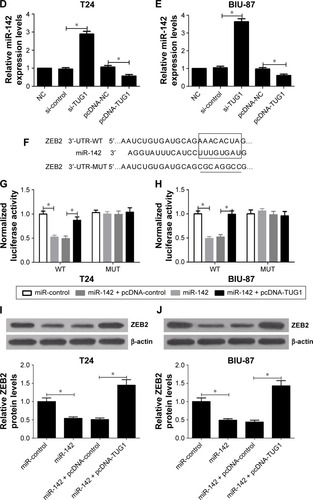
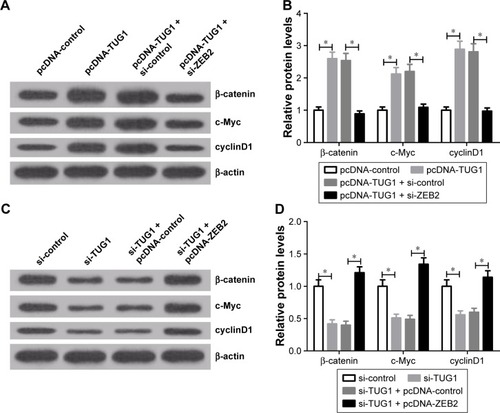
Figure 6 TUG1 regulated the Wnt/β-catenin pathway by affecting ZEB2.
Abbreviations: si, small interfering; TUG1, taurine upregulated gene 1; ZEB2, zinc finger E-box binding homeobox 2.