Figures & data
Figure 1 Proposed mechanism of action of talimogene laherparepvec. Copyright of Amgen Inc., reproduced with permission.
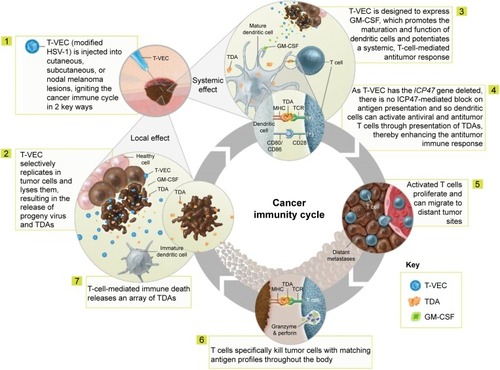
Table 1 Characteristics/modifications of talimogene laherparepvec and their impact on safety and antitumor response
Table 2 Summary of key efficacy data from OPTiM
Figure 2 Overview of the talimogene laherparepvec handling and administration process.
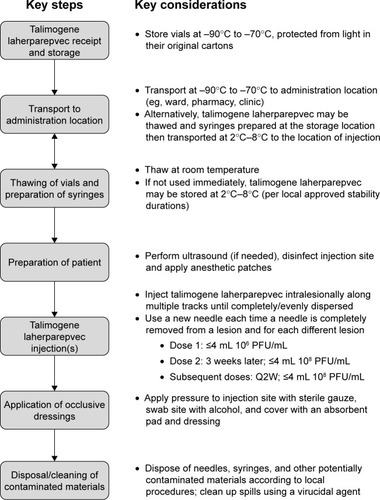
Table 3 Talimogene laherparepvec dosing schedule, including injection volumes based on lesion sizeCitation22
Table 4 Maximum storage times for talimogene laherparepvecCitation22
Figure 3 Diagram showing intralesional injection of talimogene laherparepvec into (A) cutaneous, (B) subcutaneous, and (C) nodal lesions. Copyright of Amgen Inc., reproduced with permission.Citation22
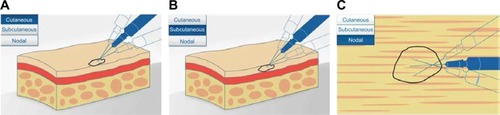
Table 5 Evaluation of response by immune-related response criteria (irRC), World Health Organization (WHO) criteria, and Response Evaluation Criteria In Solid Tumors (RECIST)
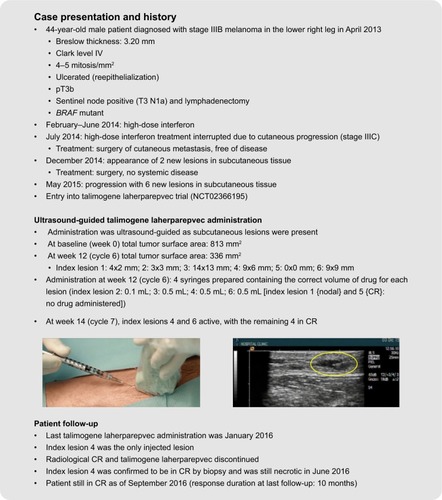
Figure 5 Case studies demonstrating standard and ultrasound-guided administration of talimogene laherparepvec. The yellow oval indicates the injected lesion under ultrasound guidance. The patients provided written informed consent for the publication of the case details and any accompanying images.