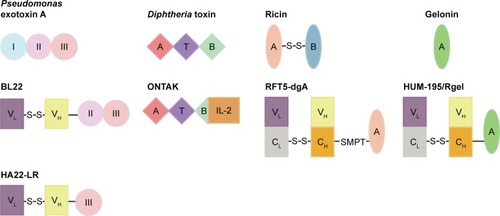
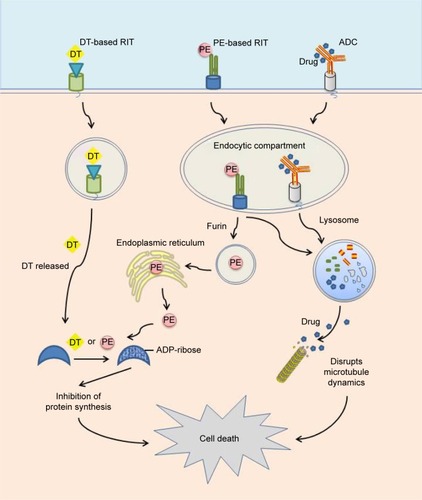
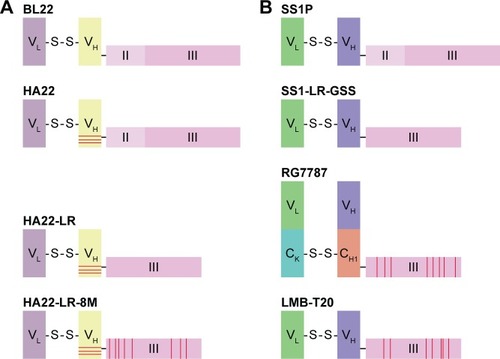
Figures & data
Table 1 RITs targeting hematologic malignancies
Table 2 RITs targeting solid tumors