Figures & data
Table 1 Patient characteristics
Table 2 Omaveloxolone attributable AEs
Table 3 All AEs, regardless of attribution
Figure 1 Safety parameters over time.
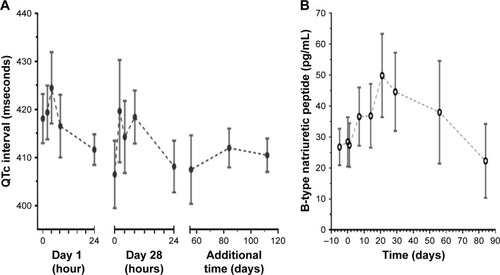
Figure 2 Pharmacokinetic assessment of omaveloxolone.
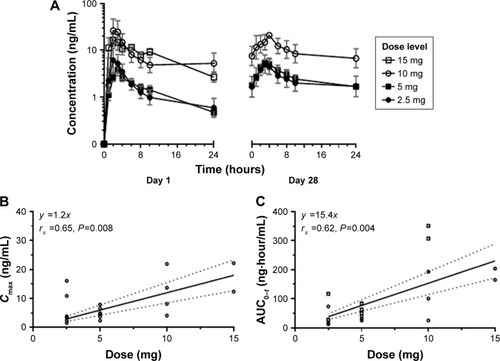
Table 4 Omaveloxolone pharmacokinetics
Figure 3 Effect of continuous oral omaveloxolone upon Nrf2 target genes.
Abbreviations: Ferritin H, ferritin heavy subunit; mRNA, messenger RNA; Nrf2, nuclear factor erythroid 2-related factor 2; PGDH, prostaglandin dehydrogenase 1.
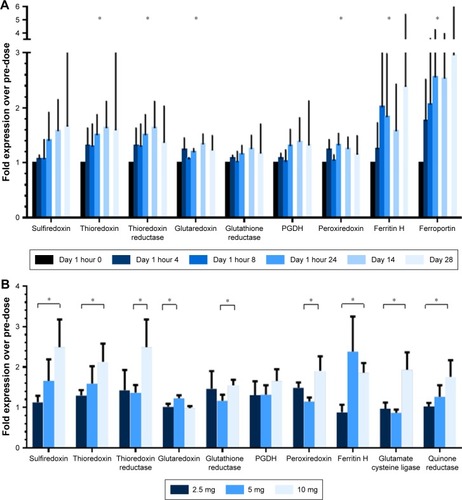
Figure 4 Representative images of NT and iNOS from limited on-treatment tumor biopsies for two patients with metastatic melanoma.
Abbreviations: iNOS, inducible isoform of nitric oxide synthase; NT, nitrotyrosine.
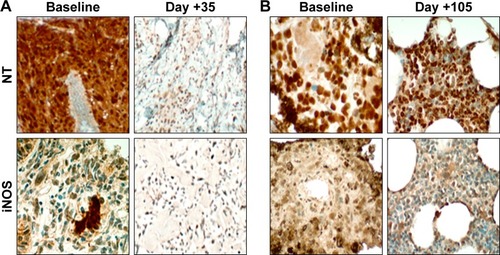
Figure 5 Decrease in peripheral blood monocytic cells over time.
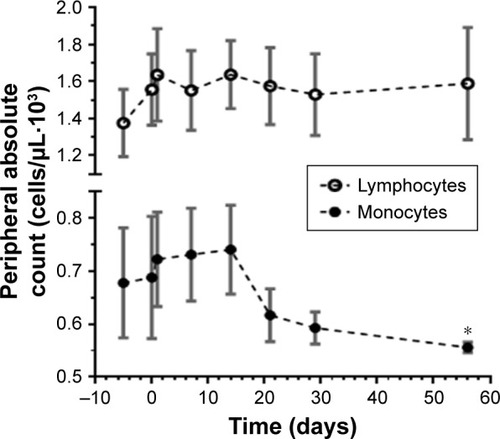
Figure 6 Patient outcome after treatment.
Abbreviations: LUSC, squamous lung cancer; LUAD, lung adenocarcinoma; MEL, melanoma; NSCLC, non-small cell lung cancer; RECIST, response evaluation criteria in solid tumor.
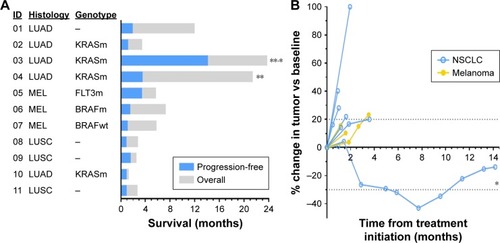
Table 5 Current clinical investigation of omaveloxolone
