Figures & data
Figure 1 Cellular proliferation by AKT phosphorylation is induced by ARID1A knockdown.
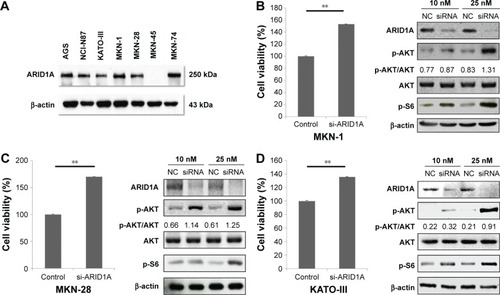
Figure 2 ARID1A depletion leads to increased sensitivity toward AKT pathway inhibitors.
Abbreviation: NC, normal control.
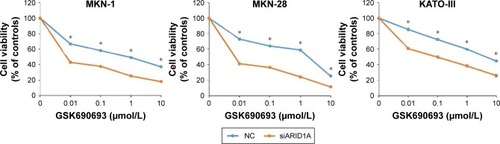
Figure 3 Loss of ARID1A expression is associated with high sensitivity to the AKT inhibitor in gastric cancer cell lines.
Abbreviation: IC50, half inhibitory concentration.
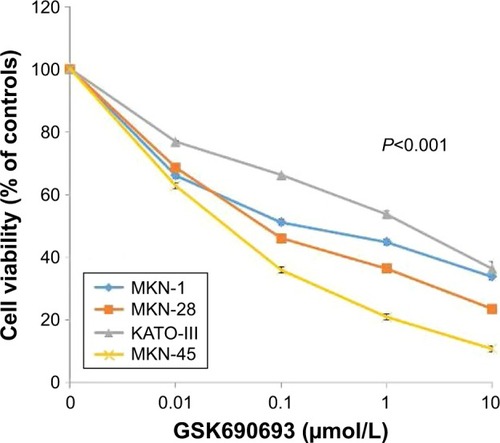
Figure 4 AKT inhibition leads to increased apoptosis in ARID1A-deficient cells.
Abbreviations: FITC, fluorescein isothiocyanate; PARP, poly-ADP ribose polymerase.
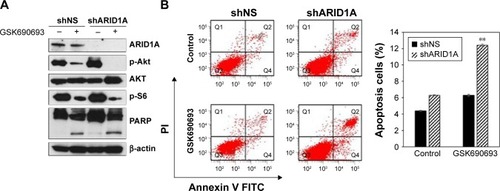
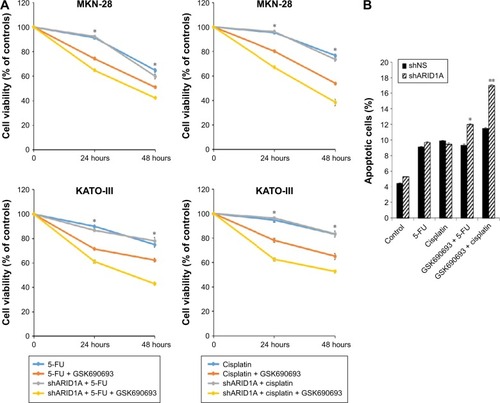
Figure 5 Loss of ARID1A expression did not induce resistance to the conventional chemotherapy.
Abbreviations: 5-FU, 5-fluorouracil; GC, gastric cancer.
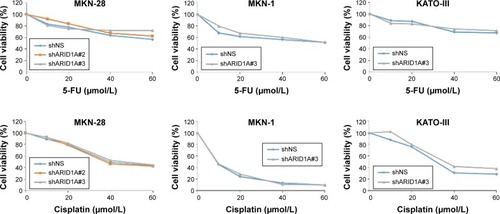
Figure 6 Addition of AKT inhibitors to conventional chemotherapy increases antitumor activity in ARID1A-deficient cancer cells.
Abbreviation: 5-FU, 5-fluorouracil.