Figures & data
Figure 1 Functional expression of CAR in Jurkat cells.
Abbreviations: APC, allophycocyanin; BSA, bovine serum albumin; CAR, chimeric antigen receptor; CEA, carcinoembryonic antigen; w/o, without.
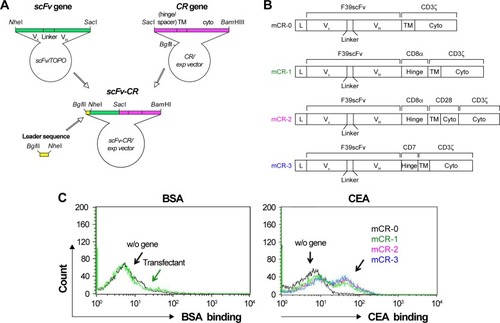
Figure 2 The scFv-IL2 fusion protein maintains the functions of both component proteins.
Abbreviations: CBB, Coomassie Brilliant Blue; CEA, carcinoembryonic antigen; FITC, fluorescein isothiocyanate; OPD, o-Phenylenediamine-2HCl; scFv, single-chain fragmented antibody; SOE, splice-overlap extension.
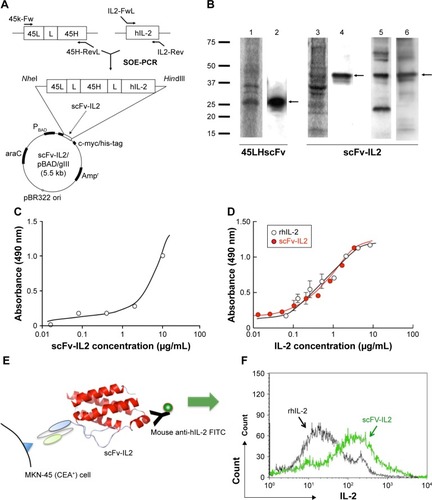
Table 1 The sequences of specific primers used for SOE-PCR of scFv-IL2
Figure 3 T cells transfected with mCR-2 were most effectively activated by MKN-45 cells.
Abbreviations: APC, allophycocyanin; BSA, bovine serum albumin; CAR, chimeric antigen receptor; CEA, carcinoembryonic antigen.
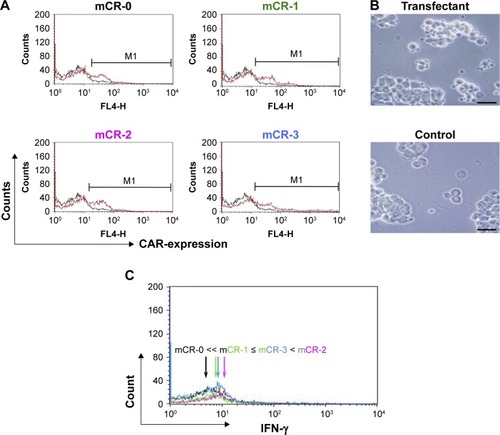
Figure 4 Combination of CAR-bearing PBMCs and scFv-IL2 enhances the antitumor effect on MKN-45 cells.
Abbreviations: APC, allophycocyanin; BSA, bovine serum albumin; CAR, chimeric antigen receptor; CEA, carcinoembryonic antigen; PBMCs, peripheral blood mononuclear cells; scFv, single-chain fragmented antibody; w/o, without.
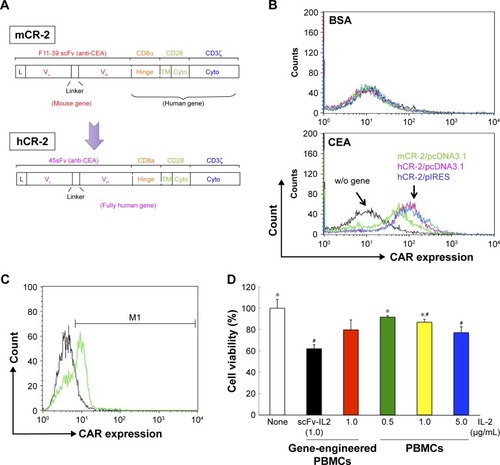
Figure S1 Artificial T-cell expansion (A) and the cell ratio of CD4+ and CD8+ T cells after 9-day expansion (B).
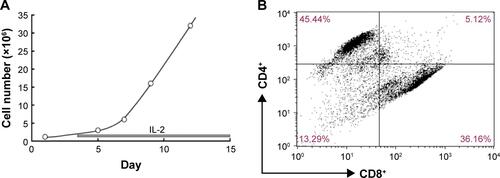
Figure S2 (A) Schematic representation of the combination of CAR-bearing PBMCs and scFv-IL2 for enhancing the antitumor activity to CEA+ tumor cells. (B) Conceptual representation of CAR-bearing PBMCs, DC, and scFv-IL2 fusion protein for enhancing the antitumor activity to cancer cells.
Abbreviations: CAR, chimeric antigen receptor; CEA, carcinoembryonic antigen; CTL, cytotoxic T lymphocytes; DC, dendric cells; NK, natural killer; PBMCs, peripheral blood mononuclear cells; scFv, single-chain fragmented antibody.
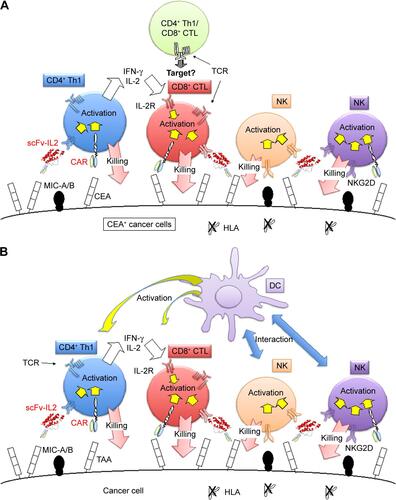
Table S1 Transfection efficiency of electroporation using Nucleofector™ and NEPA21
