Figures & data
Figure 1 SNAP-tag technology and the mechanism of action of the scFv-SNAP-AURIF ADCs.
Abbreviations: ADC, antibody–drug conjugate; BG, benzylguanine; AURIF, auristatin F; EGFR, epidermal growth factor receptor; scFv, single-chain fragment variable; VH, heavy chain variable domain; VL, light chain variable domain.
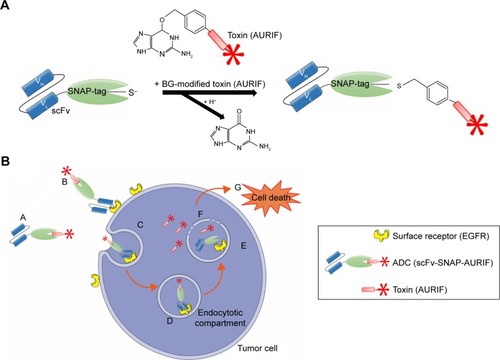
Figure 2 Generation and binding activity of scFv-SNAP-AURIF.
Abbreviations: ADC, antibody–drug conjugate; AURIF, auristatin F; BG, benzylguanine; EGFR, epidermal growth factor receptor; scFv, single-chain fragment variable; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; VG, VistaGreen.
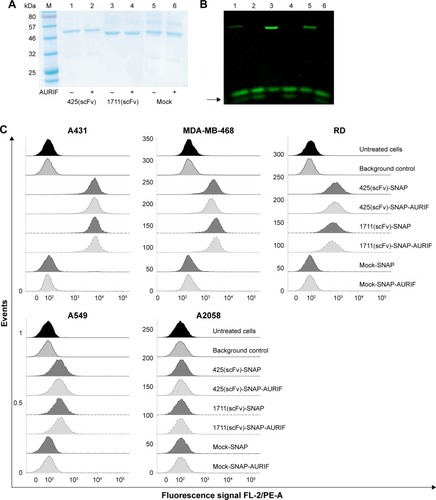
Figure 3 Cytotoxic activity of scFv-SNAP-AURIF to various solid cancer cells.
Abbreviations: ADC, antibody–drug conjugate; AURIF, auristatin F; EC50, concentration required to achieve a 50% reduction of cell viability; EGFR, epidermal growth factor receptor; PBS, phosphate-buffered saline; scFv, single-chain fragment variable.
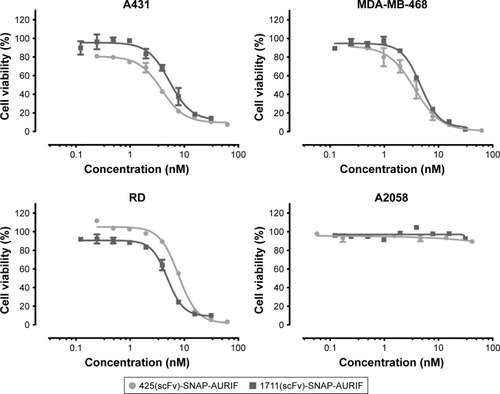
Table 1 EC50 values for AURIF and EGFR-specific ADCs
Figure 4 The effect of the EGFR-specific ADCs on cell cycle behavior.
Abbreviations: ADC, antibody–drug conjugate; ANOVA, analysis of variance; AURIF, auristatin F; EGFP, enhanced green fluorescent protein; EGFR, epidermal growth factor receptor; PBS, phosphate-buffered saline; PI, propidium iodide; scFv, single-chain fragment variable; SEM, standard error of the mean.
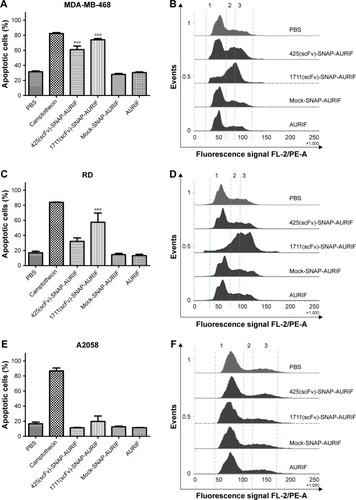
Figure 5 Cytotoxicity and microtubule dynamics assays in A549 cells.
Abbreviations: ADC, antibody–drug conjugate; AURIF, auristatin F; DAPI, 4′,6-diamidino-2-phenylindole; PBS, phosphate-buffered saline; scFv, single-chain fragment variable; TubB3, tubulin B3.
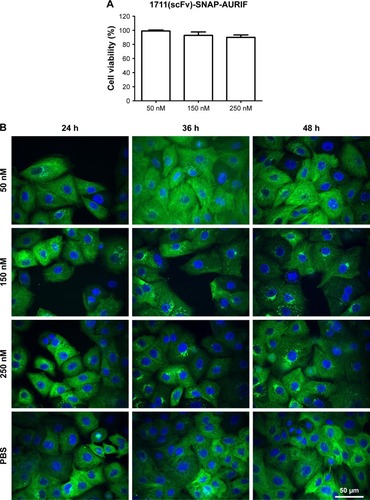
Figure 6 Specific ex vivo binding studies.
Abbreviations: ADC, antibody–drug conjugate; AURIF, auristatin F; EGFR, epidermal growth factor receptor; FFPE, formalin-fixed paraffin-embedded; scFv, single-chain fragment variable.
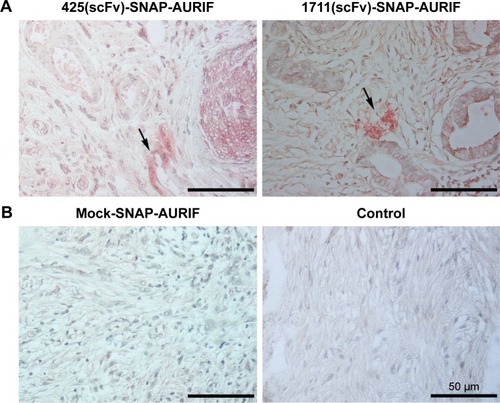
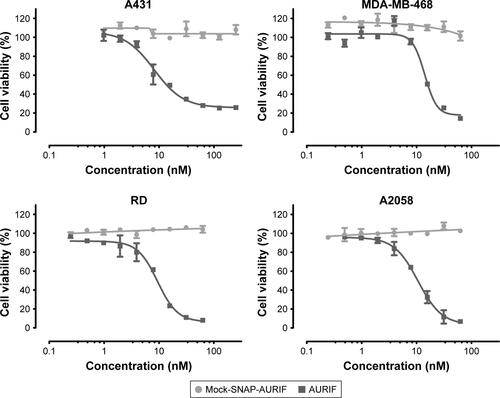
Figure S1 Cytotoxic effects of free AURIF and mock-SNAP-AURIF.
Notes: The cytotoxicity of free AURIF and mock-SNAP-AURIF assessed using an XTT-based viability assay after incubation for 72 h. Representative data from one assay are shown. Cells were incubated with decreasing concentrations of free AURIF and mock-SNAP-AURIF. The EC50 values relative to cells treated with PBS (negative control) and Zeocin (positive control) were calculated using GraphPad Prism v5. The data are shown as mean ± SD of each measurement.
Abbreviations: AURIF, auristatin F; EC50, concentration required to achieve a 50% reduction of cell viability; PBS, phosphate-buffered saline.