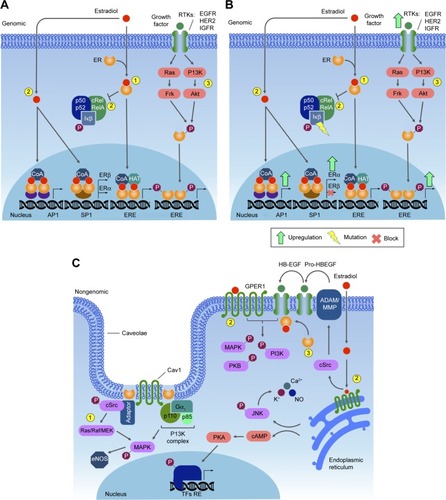
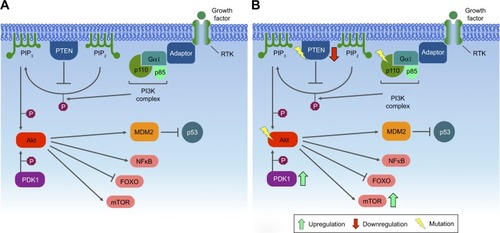
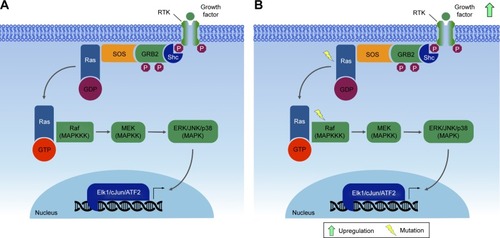
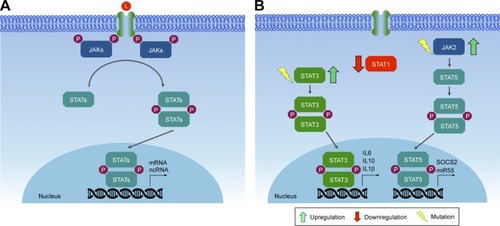
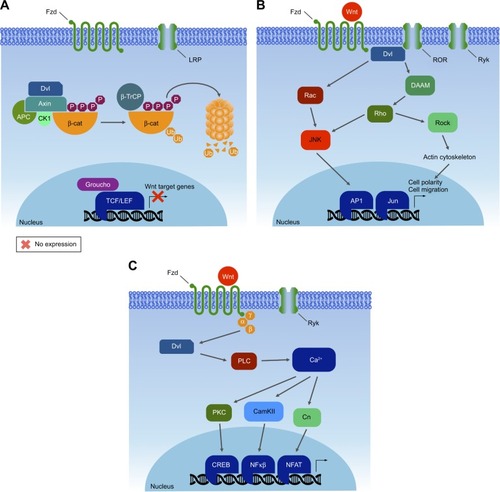
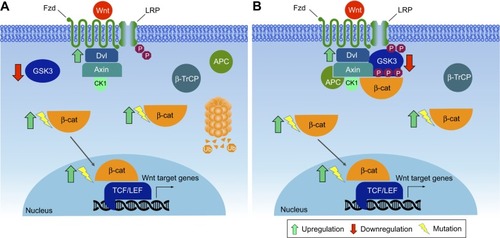
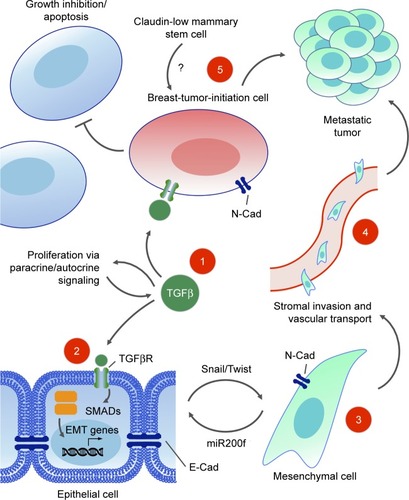
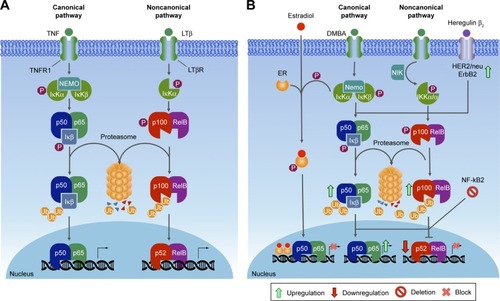
Figures & data
Table 1 Breast cancer subtype classifications, based on site of occurrence and/or biomarker status
Table 2 Main molecular alterations in pathways associated with breast cancer and respective targeted therapies