Figures & data
Figure 1 Median time to first occurrence for AEs of clinical interest in patients receiving cabozantinib during the METEOR study.
Abbreviations: AEs, adverse events; GI, gastrointestinal; IQR, interquartile range: PPES, palmar–plantar erythrodysesthesia syndrome; TE, thromboembolism; TKIs, tyrosine-kinase inhibitors.
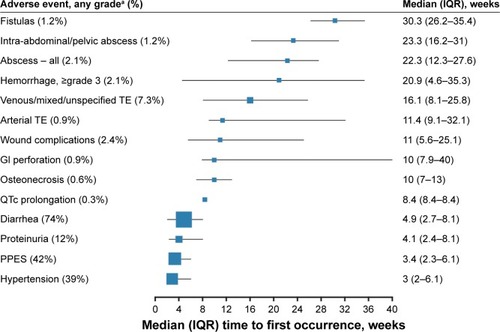
Figure 2 Cabozantinib-dosing algorithm.
Abbreviations: AEs, adverse events; GI, gastrointestinal; RPLS, reversible posterior leukoencephalopathy syndrome.
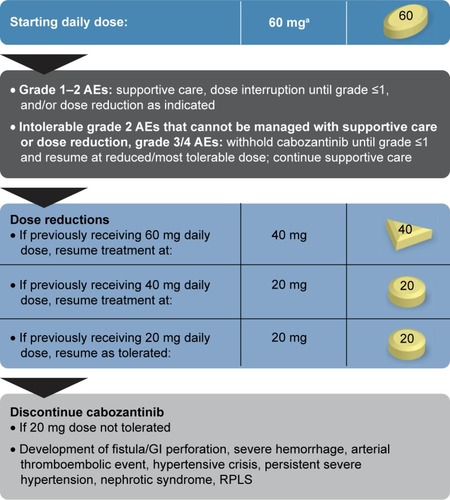
Figure 3 Common AEs experienced by patients in the cabozantinib arm of the METEOR study (safety-analysis set, n=331).Citation7
Abbreviations: ADL, activities of daily living; AE, adverse event; BP, blood pressure; Gr, grade; TPN, total parenteral nutrition; PPES, palmar–plantar erythrodysesthesia syndrome; WNL, within normal limits.
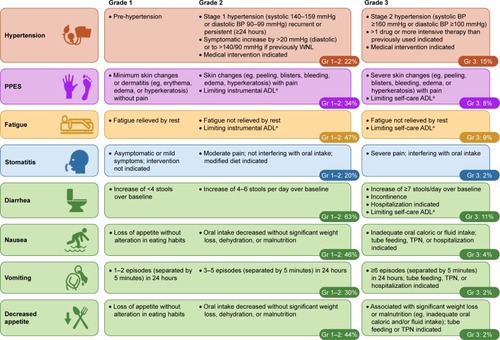
Table 1 Summary of prophylactic and supportive-care strategies for patients treated with cabozantinib
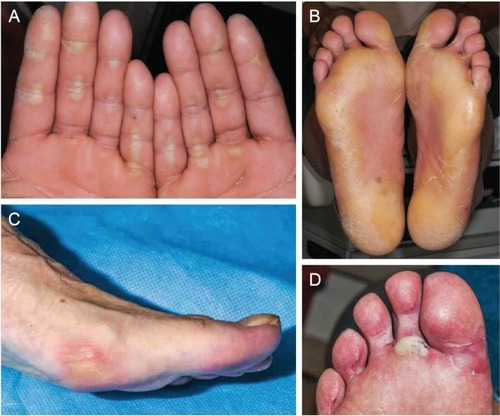
Figure 4 Examples of palmar–plantar erythrodysesthesia syndrome resulting from treatment with cabozantinib in patients with solid tumors.