Figures & data
Table 1 Summary of the characteristics of the studies included in the meta-analysis
Figure 2 Forest plot analysis of the efficiency outcomes of combined BRAF and MEK inhibition versus BRAF inhibition alone. (A) Overall response rate, (B) progression-free survival, and (C) overall survival.
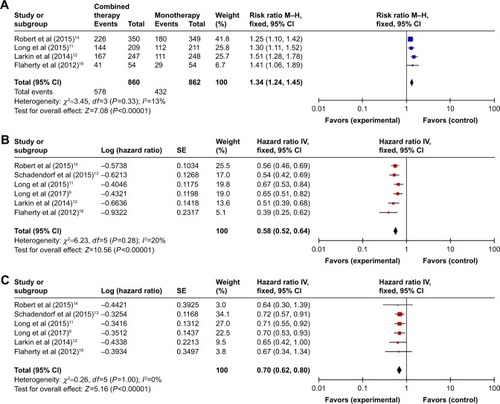
Table 2 RR of adverse events between combined targeted therapy and monotherapy
Figure 3 Subgroup analysis of the relative risk (RR) of all-grade adverse events for combined BRAF and MEK inhibition versus BRAF inhibition alone. (A) Pyrexia, (B) nausea, (C) diarrhea, (D) vomiting, and (E) arthralgia.
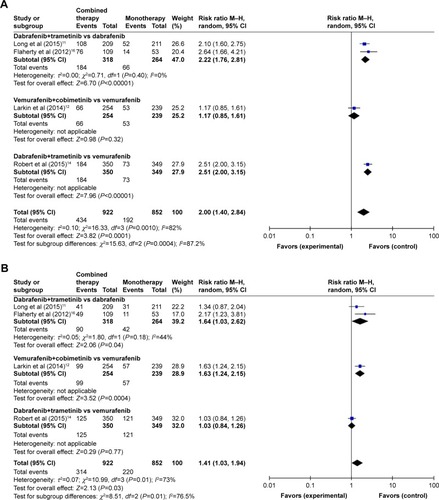
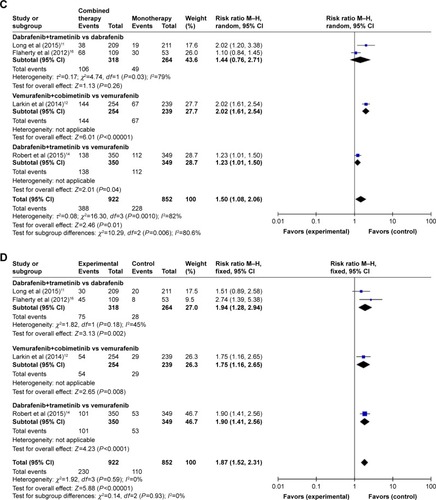
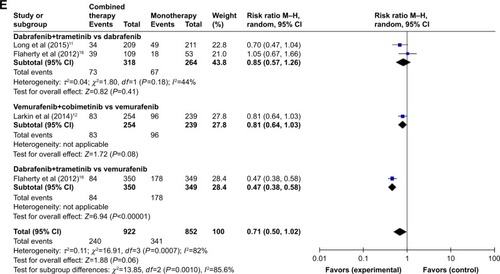
Table 3 Overall incidence of all-grade adverse events in patients receiving combined targeted therapy
Figure 4 Subgroup analysis of the incidence of all-grade adverse events for combined BRAF and MEK inhibition. (A) Pyrexia, (B) nausea, (C) diarrhea, (D) vomiting, and (E) arthralgia.
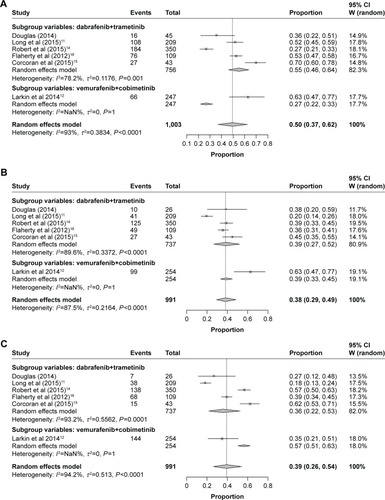
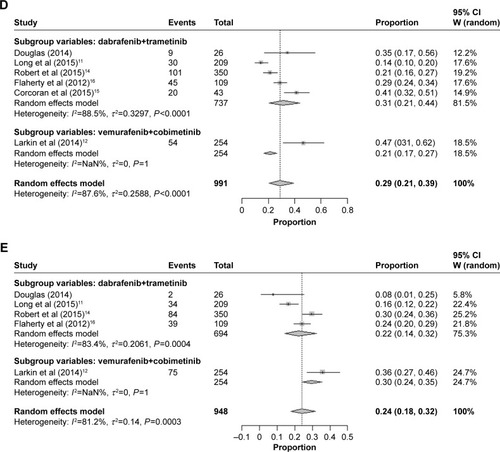
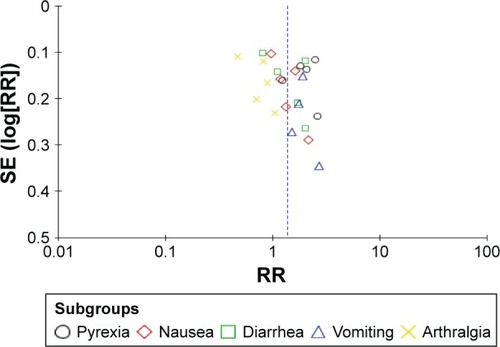
Figure 5 Funnel plot analysis for publication bias assessment.