Figures & data
Table 1 Characteristics of included studies
Figure 1 Flow diagram of the literature search and selection process.
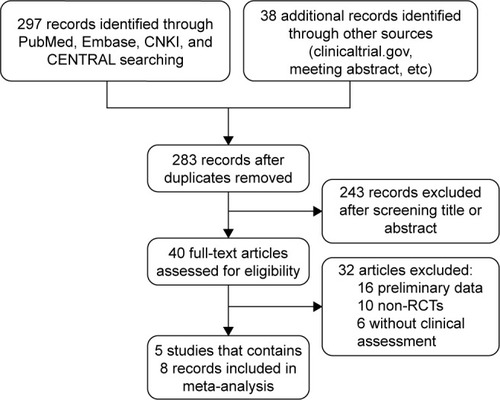
Figure 2 Risk of bias and quality assessment. (A) Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies. (B) Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
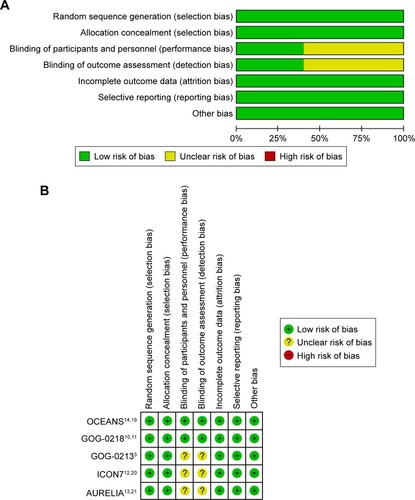
Figure 3 Forest plots of hazard ratios for progression-free survival (A and C) and overall survival (B and D). In patients with epithelial ovarian cancer treated with bevacizumab plus chemotherapy, compared with chemotherapy alone, as chemotherapy regimens of carboplatin plus paclitaxel or not (A and B). In patients with epithelial ovarian cancer with high risk of progression (FIGO stage IV disease or FIGO stage III disease and >1.0 cm of residual disease after debulking surgery) treated with bevacizumab plus chemotherapy, compared with chemotherapy alone, as chemotherapy regimens of carboplatin plus paclitaxel or not (C and D).
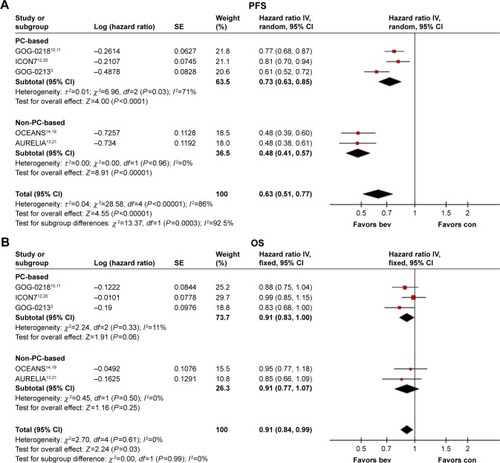
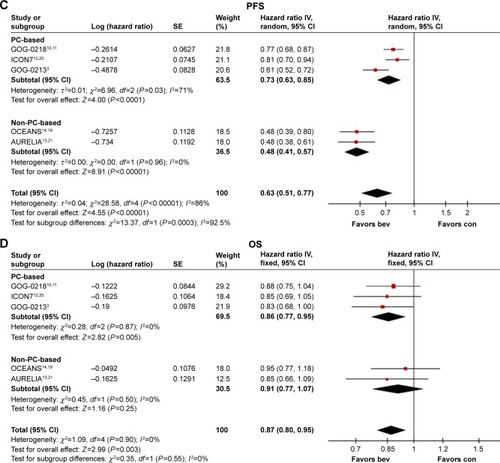
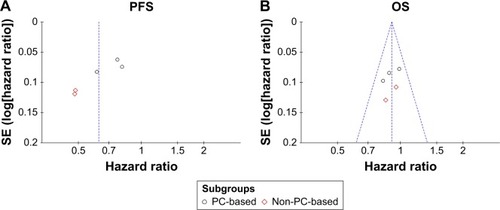
Figure 4 Funnel plots of hazard ratios for progression-free survival (A) and overall survival (B).