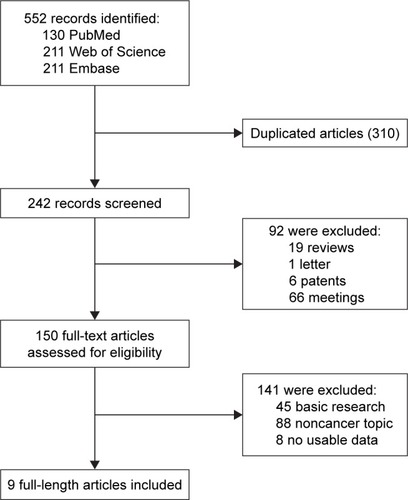
Figures & data
Table 1 Characteristics of studies included in the meta-analysis
Table 2 Subgroup analyses of pooled HRs for OS in cancer patients with abnormal expression level of DEPTOR
Figure 2 Meta-analysis of the pooled HRs of OS for cancer patients.
Abbreviation: OS, overall survival.
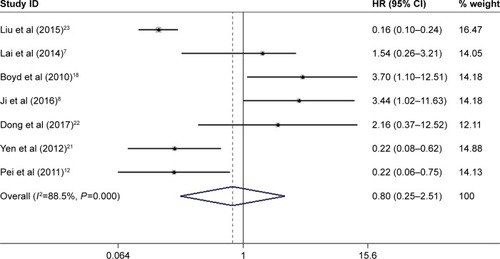
Figure 3 Results of subgroup analysis of pooled HRs of OS for cancer patients.
Abbreviations: OS, overall survival; NOS, Newcastle–Ottawa Scale.
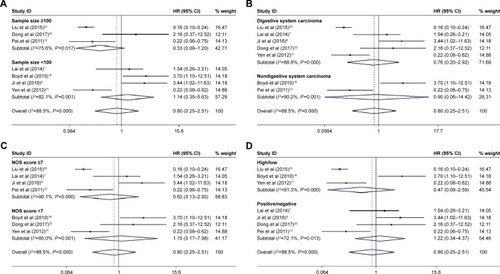
Figure 4 Meta-analysis of the pooled HRs of EFS for cancer patients.
Abbreviation: EFS, event-free survival.
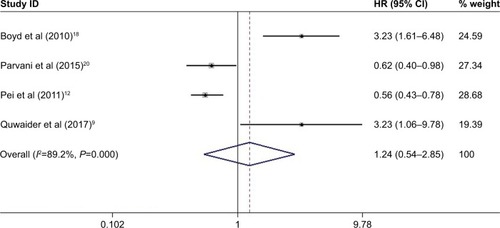
Table 3 Association between DEPTOR and clinicopathological characteristics of cancer patients
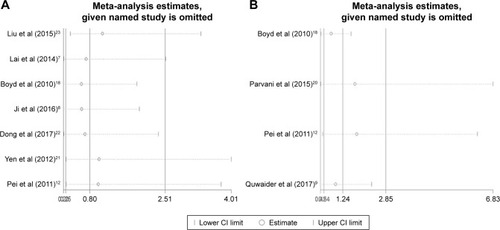
Figure 5 Sensitivity analysis plot of pooled HRs of OS (A) and EFS (B) for cancer patients with abnormally expressed DEPTOR.