Figures & data
Figure 1 Mechanisms that have been proposed to mediate the anticancer effects of chloroquine alone or in combination with other antitumor agent(s).
Notes: Up-to-date knowledge on most of the signaling pathways well known to be affected by chloroquine other than autophagy, which is a homeostatic response to various modalities or adverse environmental conditions, such as hypoxia, TACE, or exposure of malignant cells to naturally occurring or synthetic antitumor agents (see text “Preclinical data suggesting a potential for repurposing chloroquine In vitro and in vivo evidence” for details). Upward and downward orange arrows denote positive and negative regulation by chloroquine on the expression of a set of molecules (represented by a box with names of cancer-associated molecules whose changes in expression levels do not necessarily take place concurrently) or on the levels of a certain molecule, respectively.
Abbreviations: CSC, cancer stem cell; DSBs, double-strand breaks; HR, homologous recombination; NK, natural killer; TACE, transcatheter arterial chemoembolization.
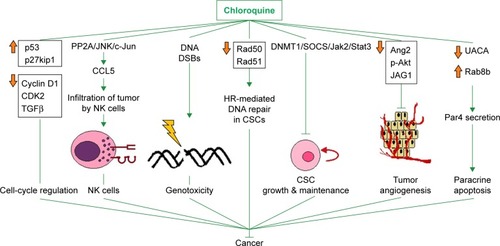
Figure 2 Octreotide targets the Axin1:APC2 interface.
Notes: (A) Octreotide (green) docked to regulators of G-protein signaling (RGS)-homologous domain (aa region 74–220) of Axin1 (orange); (B) Ser-Ala-Met-Pro (SAMP) repeat (aa region 2,035–2,050) from APC2 (magenta) in complex with Axin1 (PDB: 1EMU).
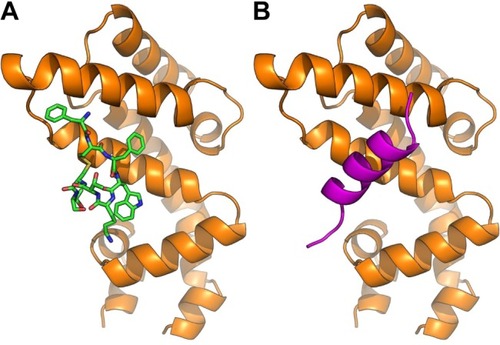
Figure 3 Octreotide targets the β-catenin:Axin1 interface.
Notes: (A) Octreotide (green) docked to armadillo repeat region (aa region 142–665) of β-catenin (gray); (B) β-catenin in complex with β-catenin binding domain (aa region 466–482) of Axin1 (orange) (PDB: 1QZ7).
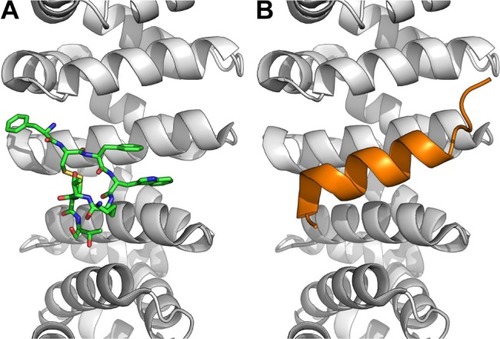
Figure 4 Octreotide targets the SENP2:SMT3C interface.
Notes: (A) Octreotide (green) docked to the catalytic domain (aa region 365–589) of SENP2 (brown); (B) SENP2 in complex with residues 20–97 of SMT3C (gray) (PDB: 1TGZ).
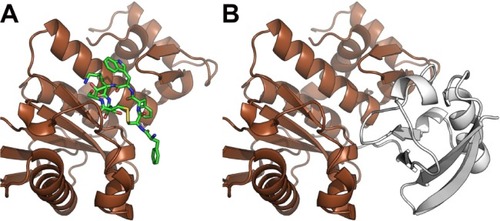
Figure 5 Octreotide targets the DKK1:LRP5 interface
Notes: (A) Octreotide (green) docked to the C-terminal domain (aa region 182–266) of DKK1 (blue). (B) DKK1 in complex with ectodomain (aa region 643–1,256) of LRP5 (red) (PDB: 3S2K).
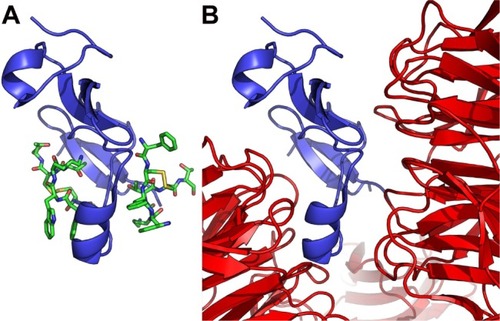
Figure 6 Octreotide targets the DKK1:Kremen1 interface.
Notes: (A) Octreotide (green) docked to residues 30–322 of Kremen1 (pink); (B) Kremen1 in complex with residues 182–266 of DKK1 (blue) (PDB: 5FWW).
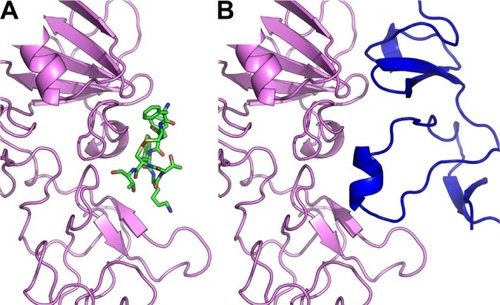
Table 1 Selected completed or ongoing clinical trials with different recruitment status that have been launched to assess chloroquine as an anticancer agent
Figure 7 Advantages of fully exploiting a marketed drug’s therapeutic potential and advantageous features of drug repositioning in oncology.
Notes: The multiyear clinical experience of the use of a marketed drug with anticancer properties not currently exploited as a direct antineoplastic agent, possible multiple therapeutic benefits (eg, octreotide exhibits both direct anticancer activities and palliates symptoms associated with bowel obstruction in patients with advanced malignancies), circumvention of the time-consuming process of launching a newly discovered pharmaceutical on the market,Citation140 as well as the well-described mechanism of action that may involve pleiotropy (eg, chloroquine may function both as a Par4 secretagogue or a DNA-repair inhibitor), in striking contrast to “targeted” anticancer bulletins that have proven to be a less promising therapeutic approach than initially expected, at least in some cases,Citation141,Citation142 are numbered among the major advantageous features of repositionable agents in comparison with newly designed/discovered agents exhibiting antitumor function. These features will possibly tilt the scales in favor of commercially available agents exhibiting antitumor function in the fight against cancer. This remains to be validated by clinical trials.
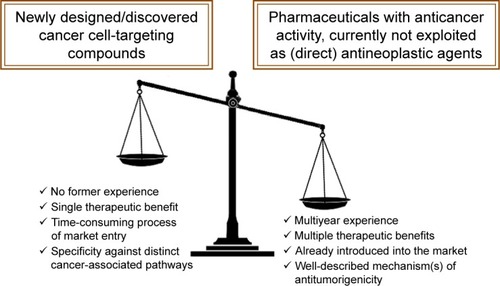
Figure S1 Chemical structure of octreotide and chloroquine.
Note: Three-letter abbreviations for amino acids have been used for simplicity.
Abbreviation: Thr(ol), threoninol (amino alcohol).
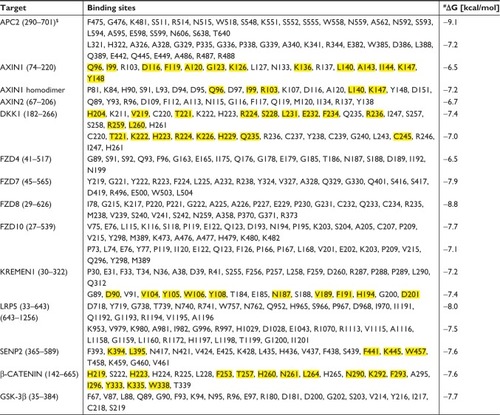
Table S1 Synopsis of direct antitumor effects of chloroquine alone or in combination with other pharmacological agents in vitro
Table S2 Composition of octreotide binding sites with corresponding affinities (ΔG).
