Figures & data
Figure 1 Combined treatment with GSK126 and Gefitinib exhibited synergic effect on survival fraction in different types of cancer cells.
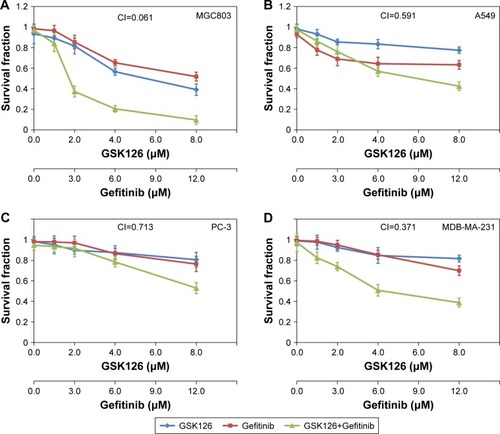
Figure 2 Combined treatment with GSK126 and Gefitinib caused enhanced apoptosis.
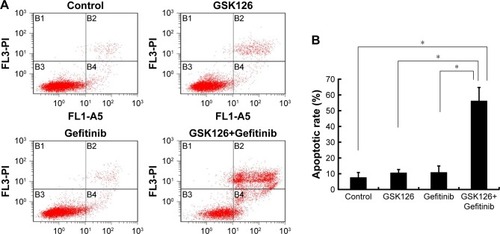
Figure 3 Combined treatment with GSK126 and Gefitinib had synergic effect on autophagy induction.
Abbreviations: 3-MA, 3-methyladenine; p-ULK, phosphorylated ULK; ULK, Unc-51-like autophagy activating kinase; t-ULK, total ULK.
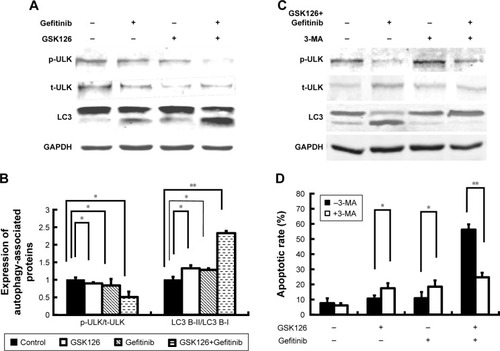
Figure 4 Combined treatment with GSK126 and Gefitinib resulted in greater inhibition of mTOR pathway.
Abbreviations: mTOR, mammalian target of rapamycin; p-mTOR, phosphorylated mTOR.
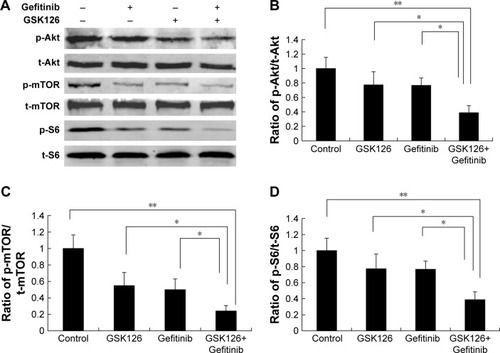
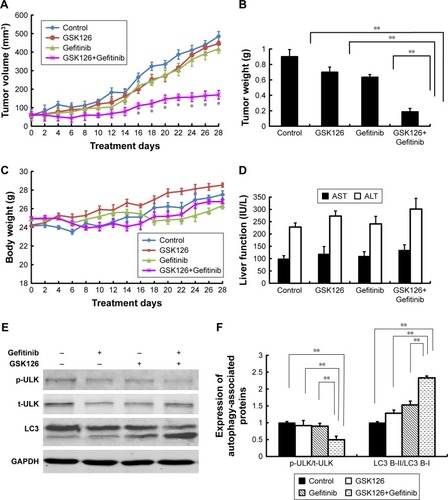
Figure 5 Combined treatment with GSK126 and Gefitinib significantly inhibited tumor xenograft in nude mice but did not affect body weight and liver function.
Abbreviations: i.p., intraperitoneal; p-ULK, phosphorylated ULK; ULK, Unc-51-like autophagy activating kinase; ALT, alanine transaminase; AST, aspartate transaminase; t-ULK, total ULK.