Figures & data
Table 1 The primers used in this work
Table 2 The loading efficiency of apatinib formulations
Figure 1 Preparation of apatinib formulations.
Abbreviations: ACN, acetonitrile; Apa-Cyc, apatinib–cyclodextrin inclusion complex; Apa-Sol, apatinib solution; ddH2O, double-distilled H2O; LC-MS/MS, liquid chromatography mass spectrometry/mass spectrometry; MRM, multi reaction monitoring.
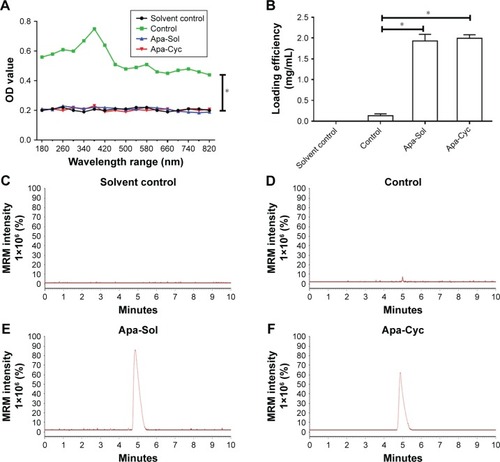
Figure 2 Antitumor effect of apatinib formulations on the subcutaneous growth of MHCC97-H cells.
Abbreviation: Apa-Cyc, apatinib–cyclodextrin inclusion complex.
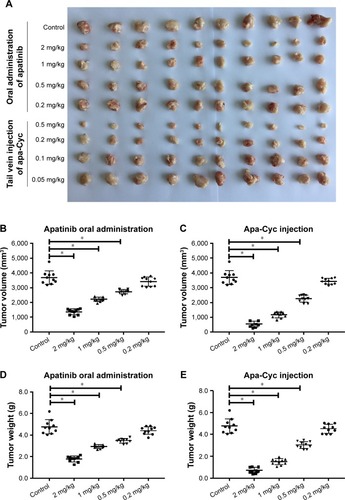
Table 3 Phase-transition temperature of Apa-Gel containing different poloxamer 407 concentrations
Figure 3 In vitro release of Apa-Gel.
Abbreviations: ACN, acetonitrile; Apa-Gel, a temperature-sensitive phase-change hydrogel of apatinib; ddH2O, double-distilled H2O; LC-MS/MS, liquid chromatograph mass spectrometry/mass spectrometry; MRM, multi reaction monitoring.
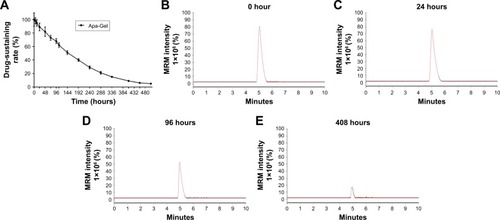
Table 4 Half-life of apatinib in HCC tissues injected with apatinib formulations
Figure 4 In vivo release of apatinib formulations in HCC tumor tissues.
Abbreviations: ACN, acetonitrile; Apa-Gel, a temperature-sensitive phase-change hydrogel of apatinib; Apa-Sol, apatinib solution; Apa-Cyc, apatinib–cyclodextrin inclusion complex; LC-MS/MS, liquid chromatography mass spectrometry/mass spectometry.
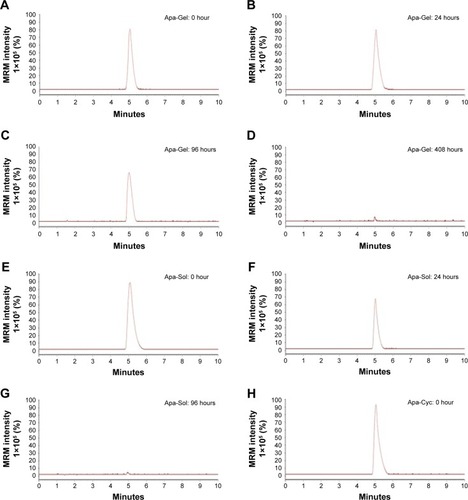
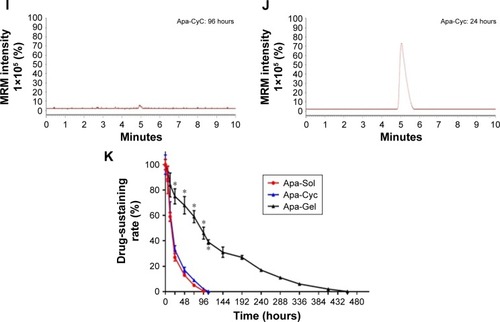
Figure 5 Long-acting antitumor effect of Apa-Gel formulations on the subcutaneous growth of MHCC97-H cells.
Abbreviations: Apa-Gel, a temperature-sensitive phase-change hydrogel of apatinib; Apa-Sol, apatinib solution; Apa-Cyc, apatinib–cyclodextrin inclusion complex.
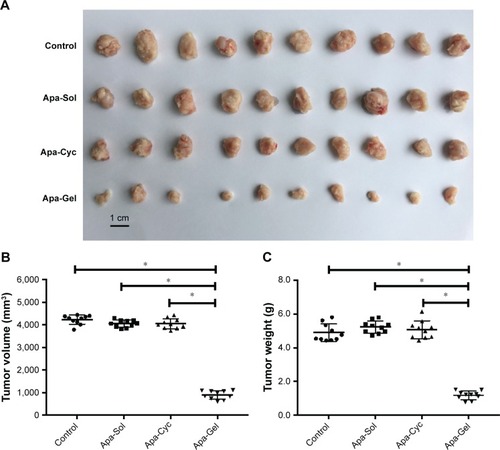
Figure 6 Long-acting antitumor effect of Apa-Gel formulations on the EMT process of MHCC97-H cells.
Abbreviations: Apa-Cyc, apatinib–cyclodextrin inclusion complex; Apa-Gel, a temperature-sensitive phase-change hydrogel of apatinib; Apa-Sol, apatinib solution; EMT, epithelial–mesenchymal transition; qPCR, quantitative polymerase chain reaction.
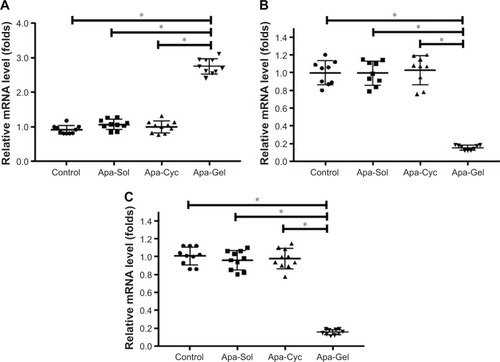
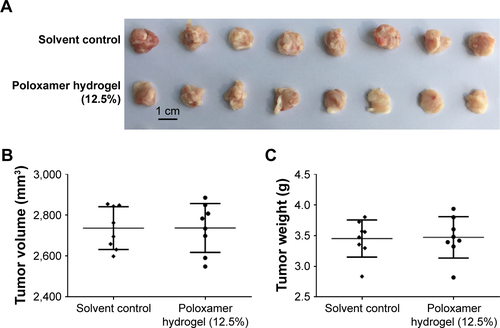
Figure S1 Poloxamer 407 hydrogel did not inhibit subcutaneous growth of MHCC97-H in nude mice.