Figures & data
Table 1 Baseline clinical characteristics and relationship with pCR for enrolled HER2 positive breast cancer patients
Table 2 MetS-related variables at initiation of NAT and pCR in HER2 positive breast cancer patients
Table 3 MetS-related variables change before and after NAT in HER2 positive breast cancer patients
Table 4 Multivariate logistic regression analysis of factors associated with pCR in HER2 positive breast cancer patients
Table 5 Multivariate logistic regression analysis of factors associated with pCR in HER2 positive breast cancer cases receiving neoadjuvant targeted therapy
Figure 1 Distribution of IGF-1 levels before and after neoadjuvant therapy and its association with pCR rate in HER2 breast cancer patients. Those achieving pCR were presented in violet squares, and those without pCR were presented in blue circles. The median value of 178.00 ng/mL was adopted to classify patients into higher or lower IGF-1 expression. Patients with lower IGF-1 levels both pre and post NAT were statistically more likely to achieve pCR compared with others (P=0.012).
Abbreviations: IGF-1, insulin-like growth factor-1; NAT, neoadjuvant therapy; pCR, pathological complete response.
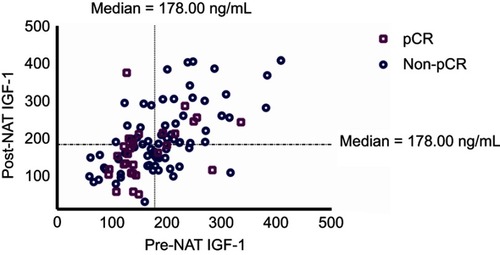
Figure 2 Disease free survival of enrolled patients by pCR and IGF-1 status. The median value of 178.00 ng/mL was adopted to classify patients into higher or lower IGF-1 expression. At a median follow up time of 24.50 (range 4.57–53.63) months, a total of 15 disease-free events were reported. The number of DFS events were relatively fewer in the lower IGF-1 group. DFS rate was similar between (A) pCR and non-pCR groups (P=0.166), (B) lower and higher IGF-1 groups in whole study population (P=0.288), or (C) lower and higher IGF-1 groups in non-pCR patients (P=0.265).
Abbreviations: DFS, disease-free survival; pCR, pathological complete response; IGF-1, insulin-like growth factor-1.

Table 6 Multivariate analysis of prognostic factors affecting DFS in HER2 positive breast cancer patients treated with neoadjuvant therapy
