Figures & data
Table 1 Clinicopathological characteristics of 31 patients with endometrial cancer
Figure 1 Upregulated NLPR3 inflammasome activation is associated with the progression of human endometrial cancer. (A–C) The expression NLPR3 in 31 endometrial cancer and paired adjacent non-tumor tissues were examined by immunohistochemistry, quantitative PCR, and Western blot. (D–E) Stratification analysis of the association of NLRP3 expression with cancer stage and grade. (F–I) Quantitative PCR analysis of ASC, caspase-1, IL-1β, and IL-18 mRNA levels in endometrial cancer and adjacent non-tumor endometrial tissues. Data are representative images or expressed as individual values or median ±75% percentile (magnification ×400). *P<0.05, **P<0.01, ***P<0.001.
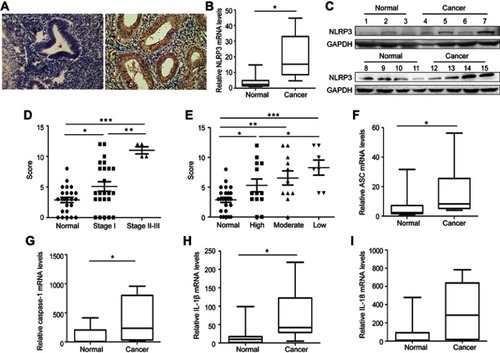
Figure 2 Altered NLPR3 expression modulated the proliferation, clonogenicity, migration, and invasion in endometrial cancer cells. Ishikawa and HEC-1A cells were transduced with lentivirus for NLPR3 silencing. (A) Western blot analysis of relative NLRP3 levels in NLRP3 silence cells. The proliferation (B), clonogenicity (C), invasion (D), and wound healing (E) in different groups of cells were determined. Ishikawa and HEC-1A cells were transduced with lentivirus for NLPR3 overexpression. (F) Western blot analysis of relative NLRP3 levels in NLRP3 overexpressing cells. The relative levels of NLPR3, IL-1β, and caspase-1 were determined by Western blot. Data are representative images or expressed as mean ± SEM of each group from three separate experiments. The proliferation (F), migration (mig, G), and invasion (inva, H) in different groups of cells were determined. (I) Caspase-1 inhibitor YVAD-cmk inhibits the proliferation of endometrial cells. *P<0.05, **P<0.01, ***P<0.001.
Abbreviations: ISK, Ishikawa; Casp-1, Caspase-1.
Figure 3 NLRP3 silencing suppressed the growth of xenotransplant tumors in nude mice. BALB/c nude mice were implanted subcutaneously with Ishikawa/mock or Ishikawa/shNLPR3 cells into their left upper flank. At the end of the experiment, the mice were sacrificed and tumors were dissected (A). Tumor growth curve was monitored (B). Dissected tumors were weighted (C). The levels of Ki67, VEGF, NLPR3, caspase-1, pro-IL-1β, and IL-1β expression in the tumor tissues were determined by immunohistochemistry (scale bar: 50 µm) and Western blot (D–F). Data are representative images or expressed as mean ± SEM of each group. *P<0.05, **P<0.01, ***P<0.001.
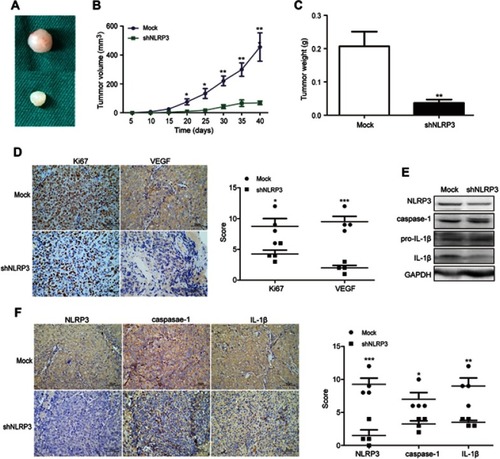
Table 2 Expression of NLRP3 and ERβ in endometrial cancer tissues
Table 3 NLRP3 expression is positively associated with ERβ expression
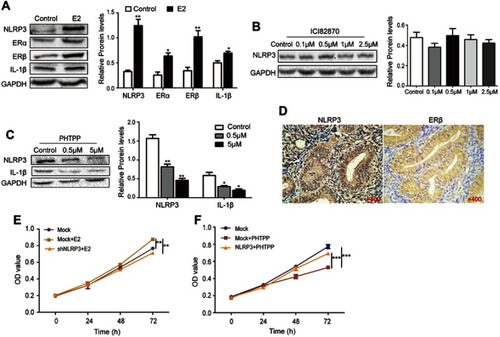
Figure 4 Estrogen enhanced NLPR3 inflammasome activation and endometrial cancer cell proliferation in vitro. (A–C) Ishikawa cells were treated with estrogen, ICI182870 or PHTPP for 24 hrs and relative levels of NLPR3, ERα, ERβ, IL-1β were determined by Western blot. (D) The expression of NLPR3 and ERβ in human endometrial cancer tissues was examined by immunohistochemistry (magnification ×400). (E) Estrogen promoted the proliferation of Ishikawa cells and NLRP3 silencing dramatically reversed the effects. (F) Blockage of ERβ inhibited the proliferation of Ishikawa cells and NLRP3 overexpression dramatically reversed the effects. Data are representative images or expressed as mean ± SEM of each group. *P<0.05, **P<0.01, ***P<0.001.