Figures & data
Figure 1 Dioscin inhibited colorectal cancer proliferation and colony formations. (A) The chemical structure of dioscin; (B–D) Dioscin inhibited colorectal cancer proliferation in vitro. HT-29 (B), HCT-116 (C) and SW-480 (D) cells were placed into 96-well plates and then treated with different concentrations of dioscin for 24, 48, 72 hrs, respectively, the cell viability was measured by the Cell Titer-Glo kit as described. (E–G) Dioscin inhibited the colony formation of colorectal cells. HT-29 (E), HCT-116 (F) and SW-480 (G) cell suspensions treated with dioscin were plated into 6-well plate, and the colony formation was examined as described in methods. Left, the representative images; right, quantitative statistics expressed as mean ± SD. *p<0.05, **p<0.01, ***p<0.001 versus the control.
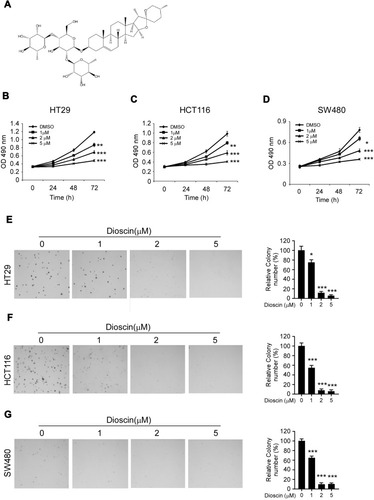
Figure 2 Dioscin inhibited tumor glycolysis in colorectal cancers by downregulating hexokinase-2. (A) The expression of hexokinase-2 in colorectal cancer tissue and paired adjacent tissue was examined by IHC staining. Left, the representative images; right, the statistics of hexokinase-2 expression. ***p<0.001 indicated a significant difference. (B) The expression of hexokinase-2 in normal colon cells and colorectal cancer cells was examined by Western blotting. (C–E) HT-29 (C), HCT-116 (D) and SW-480 (E) cells were treated with dioscin and the expression of hexokinase-2 (left), glucose consumption (middle) or lactate production (right) was measured, respectively. (F–G) Hexokinase-2 overexpression impaired dioscin-induced glycolysis inhibition. Ectopic overexpression of hexokinase-2 was performed in HCT-116 and HT-29 cells and then treated with 5 μM dioscin, the amount of glucose (F) and lactate (G) were measured as described. *p<0.05, **p<0.01, ***p<0.001 versus the control.
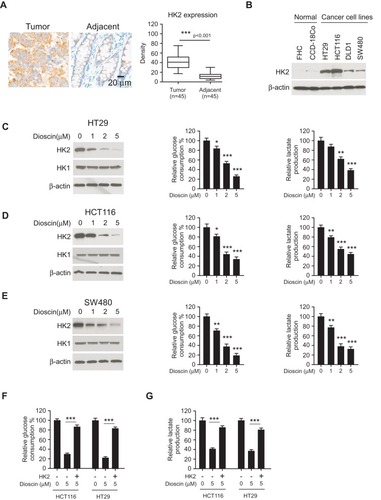
Figure 3 Dioscin-induced cell apoptosis in colorectal cancer via suppressing hexokinase-2. (A, B) Dioscin impaired the interaction between hexokinase-2 and VDAC-1. HCT-116 (A) or HT-29 (B) Cells were treated with 1μM dioscin for 24 hrs, and the cell lysates were harvested. The cell lysates were immunoprecipitated with anti-hexokinase-2 or anti-VDAC-1 antibodies, respectively, and then probed by Western blotting with indicated antibodies. (C, D), Dioscin induced the translocation of cytochrome C and Bax. HCT-116 (C) or HT-29 (D) cells were treated with the indicated concentration of dioscin, the cytosolic and mitochondria fractions were extracted as described, respectively, and then examined with indicated antibodies. (E, F) Dioscin-induced cell apoptosis in CRC cells. HT-29, HCT-116, or SW480 cells were treated with dioscin for 24 hrs, cleaved PARP and caspase-3 (E) or the activity of caspase-3 (F) was examined. (G, H) Ectopic overexpression attenuated dioscin-induced cell apoptosis. HT-29 or HCT-116 cells were transfected with plasmid overexpressing hexokinase-2 and then treated with 5 μM dioscin, and the expression of cleaved PARP and caspase-3 (G) or the activity of caspase-3 (H) was measured. *p<0.05, **p<0.01, ***p<0.001 represented significant differences.
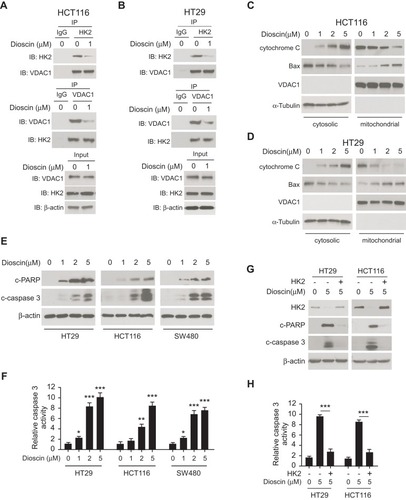
Figure 4 Dioscin inhibited hexokinase-2 expression by downregulating c-myc. (A, B) c-myc knockdown suppressed hexokinase-2 expression and tumor glycolysis. HCT-116 (A) or HT-29 (B) cells were transfected with c-myc shRNA, the expression of hexokinase-2 in transfected cells (left), glucose consumption (middle) or lactate production (right) was examined. (C) Dioscin reduced c-myc expression in CRC cells. CRC cells were treated with indicated concentrations of dioscin, and the expression of c-myc was detected. (D) Exogenous overexpression of c-myc attenuated hexokinase-2 suppression by dioscin. HCT-116 cells were transfected with plasmids overexpressing c-myc and then treated with 5μM dioscin, the expression of c-myc and hexokinase-2 was detected. (E, F) c-myc ectopic overexpression attenuated dioscin-induced cell apoptosis. HCT-116 cells were transfected with plasmid overexpressing c-myc and then treated with 5μM dioscin, and the expression of HK-2, cleaved PARP and caspase-3 (E) or the activity of caspase-3 (F) was measured. **p<0.01, ***p<0.001 represented significant differences.
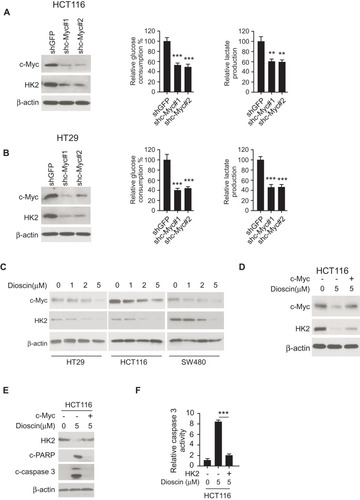
Figure 5 Dioscin promoted the ubiquitination of c-myc. (A) Proteasome inhibitor MG132 antagonized dioscin-mediated c-myc reduction. CRC cell was pretreated with 20 μM MG132 and then treated with 5μM dioscin; the expression of c-myc was examined by Western blotting. (B) Dioscin increases the ubiquitination levels of c-myc. HCT-116 cells were pretreated with 20 μM MG132 and then incubated with indicated dioscin, the cell lysates were immunoprecipitated with anti-c-myc antibody and then probed with anti-ubiquitin antibody. (C) FBW-7 was involved in dioscin-mediated ubiquitination of c-myc. HCT-116 were transfected with FBW7 siRNA and then treated with 5μM dioscin, the ubiquitination of c-myc was detected as described. (D) Dioscin enhanced the binding between c-myc and FBW-7. HCT-116 cells were treated with 5μM dioscin, the cell lysates were immunoprecipitated with anti-FBW7 antibody, and the precipitation was probed with the anti-c-Myc antibody.
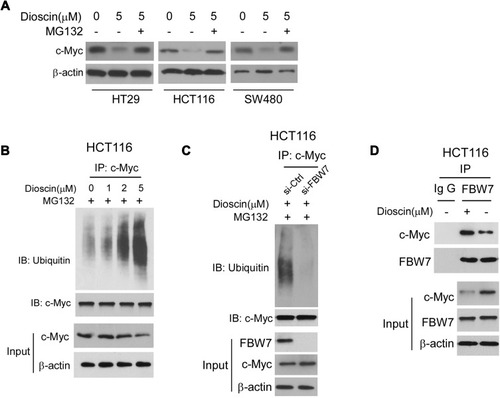
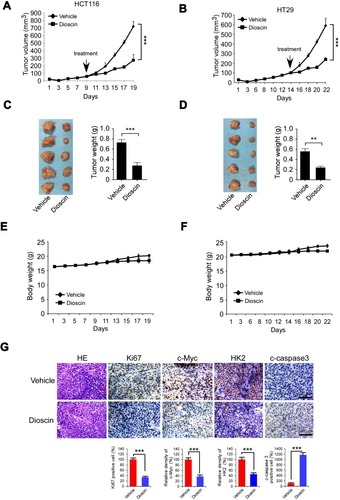
Figure 6 Disocin inhibited CRC xenograft growth in nude mice. (A–D) Dioscin restrained the growth of HCT-116 and HT-29 xenografts. The nude mice bearing HCT-116 or HT-29 xenografts were administrated with dioscin, and the growth rate of HCT-116 (A) or HT-29 (C) xenograft was measured. At the end of experiments, HCT-116 (B) or HT-29 (D) xenograft was isolated, photography, and weighed. Left: the images of tumor tissue; right: the weight of tumor tissue expressed as mean±SD. **p<0.01, ***p<0.001 versus the vehicle. (E–F) The curve of the body weight of nude mice bearing HCT-116 (E) or HT-29 (F) tumors during the experiments. (G) The expression of Ki67, c-myc, hexokinase-2 and cleaved-caspase-3 was decreased after dioscin treatment. In the end of in vivo experiments, tumor tissue was collected and stained with Ki67, c-myc, hexokinase-2 and cleaved caspase-3 antibodies, respectively. Upper, the representative photograph. Below, the expression was quantified, ***p<0.001 indicated a significant difference.