Figures & data
Table 1 Relationship Between Clinico-Pathological Characteristics and MCTP1-AS1 Expression in EC Patients
Figure 1 MCTP1-AS1 is downregulated in EC tissues and cell lines and correlated with prognosis in EC patients. (A) Expression level of MCTP1-AS1 is downregulated in endometrial cancer tissues compared with adjacent normal tissues (n=60, ****p<0.0001). (B) The overall survival rate is significantly lower in patients with low MCTP1-AS1 expression than in those with high MCTP1-AS1 expression. n=30 for each group. (C) Expression levels of MCTP1-AS1 in EC cell lines compared to the normal esophageal epithelial cells. Data are mean ± SD of triplicate experiment (**p<0.01).
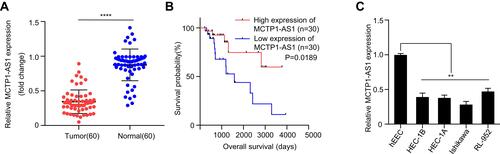
Figure 2 MCTP1-AS1 inhibits cell proliferation, cell migration, cell invasion and EMT process in EC cells. (A) The transfection efficiency of Ishikawa cells in response to overexpression MCTP1-AS1 plasmid, as detected by quantitative RT-PCR (**p<0.01). (B) Cell proliferation was measured in Ishikawa cells overexpressed MCTP1-AS1 plasmid using CCK8 at 0 h, 24 h, 48 h and 72 h time points. Data are mean ± SD of triplicate experiment (**p<0.01). (C) Wound healing assay was performed in both cell lines following transfection with MCTP-AS1 overexpression plasmid. (D) Transwell assay was used to measure the effect of MCTP1-AS1 overexpression on cell migration and invasion in Ishikawa cells. Data are mean ± SD of triplicate experiment (**p<0.01). (E) MCTP1-AS1 overexpression significantly promotes the expression of E-cadherin but inhibited N-cadherin and vimentin.
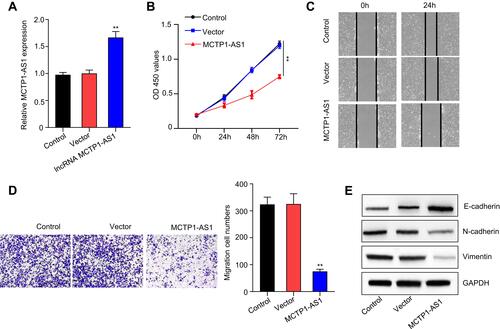
Figure 3 MCTP1-AS1 reversely regulated miR-650 expression in EC cells by directly targeting. (A) The binding scheme of MCTP1-AS1 and miR-650. (B) MCTP1-AS1 interacts with miR-513 by directly targeting verified by luciferase reporter assay. Data are mean ± SD of triplicate experiment (**p<0.01). (C) The direct binding of MCTP-AS1 and miR-650 was measured by RIP assay. (D) miR-650 expression levels are significantly increased in human EC tissues compared with adjacent normal tissues. Data are mean ± SD of triplicate experiment (**p<0.01, n=60). (E) An inverse correlation is observed between mRNA expressions of miR-650 and MCTP1-AS1 in human EC tissues. n=60. p<0.0001. (F) Expression levels of miR-650 in EC cell lines compared to the normal esophageal epithelial cells. Data are mean ± SD of triplicate experiment (**p<0.01). (G) Decreased miR-650 expression in Ishikawa cells in response to overexpression MCTP1-AS1 plasmid, as detected by quantitative RT-PCR. Data are mean ± SD of triplicate experiment (**p<0.01).
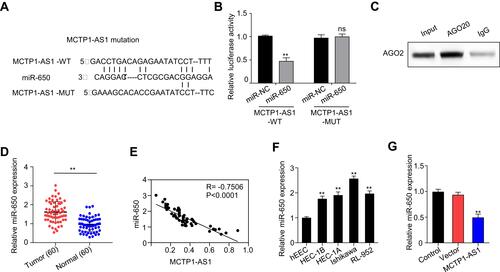
Figure 4 MCTP1-AS1 regulated EMT process via miR-650 in EC cells. (A) miR-650 mimic was transfected into Ishikawa cells, and the transfection efficiency was verified using qRT-PCR. Data are mean ± SD of triplicate experiment (**p<0.01). (B) Cell proliferation was measured in MCTP1-AS1 overexpressed Ishikawa cells transfected with miR-650 mimic using CCK8 at 0 h, 24 h, 48 h and 72 h time points. Data are mean ± SD of triplicate experiment (**p<0.01). (C) Wound healing assay was performed in MCTP1-AS1 overexpressed Ishikawa cell lines following transfection with miR-650 mimic. (D) Transwell assay was used to measure the effect of miR-650 overexpression on cell migration and invasion in MCTP1-AS1 overexpressed Ishikawa cells. Data are mean ± SD of triplicate experiment (**p<0.01). (E) Western blot shows miR-650 overexpression significantly reverses the effect of MCTP1-AS1 on E-cadherin, N-cadherin and Vimentin expression in EC cells.
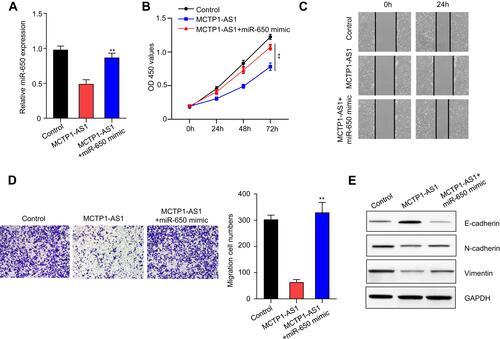
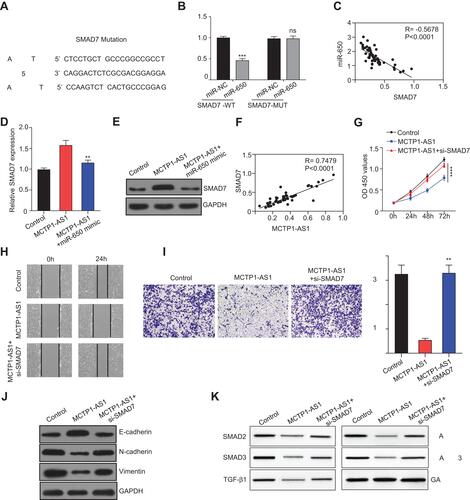
Figure 5 MCTP1-AS1 regulated SMAD7 expression via miR-650 in EC cells. (A) The binding scheme of miR-650 and SMAD7. (B) miR-650 interacts with SMAD7 by directly targeting verified by luciferase reporter assay. Data are mean ± SD of triplicate experiment (***p<0.001). (C) An inverse correlation is observed between mRNA expressions of miR-650 and SMAD7 in human EC tissues (n=60, p<0.0001). (D) qRT-PCR shows that mRNA expression level of SMAD7 is downregulated following miR-650 overexpression. Data are mean ± SD of triplicate experiment (**p<0.01). (E) Western blot shows that protein expression level of SMAD7 is downregulated following miR-650 overexpression. (F) A positive correlation is observed between mRNA expressions of MCTP1-AS1 and SMAD7 in human EC tissues (n=60, p<0.0001). (G) Si-SMAD7 is transfected into MCTP-AS1 overexpressed EC cells. CCK8 assay was used to determine the cell proliferation. Data are mean ± SD of triplicate experiment (****p<0.0001). (H) Wound healing assay was performed in MCTP1-AS1 overexpressed Ishikawa cell lines following transfection with si-SMAD7. (I) Transwell assay was used to measure the effect of si-SMAD7 transfection on cell migration and invasion in MCTP1-AS1 overexpressed Ishikawa cells. Data are mean ± SD of triplicate experiment (**p<0.01). (J) Western blot shows si-SMAD7 transfection significantly reverses the effect of MCTP1-AS1 on E-cadherin, N-cadherin and Vimentin expression in EC cells. (K) Western blot shows activation of TGF-β/SMAD pathway after transfecting si-SMAD7 in EC cells.