Figures & data
Figure 1 The CT scan of the patients before surgery. (A) The 4 cm tumor on the right upper lobe (Red arrow); (B) Metastatic right No.4 lymph node (Red arrow).
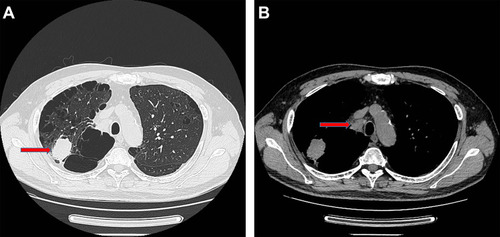
Figure 2 Immunohistochemical staining. (A) Hematoxylin-eosin staining of the tumor tissue (40X and 400X); (B) Immunohistochemical staining for Synaptophysin (Positive, 400X); (C) Immunohistochemical staining for Chromogranin A (Positive, 400X); (D) Immunohistochemical staining for Ki-67 (80% Positive, 400X).
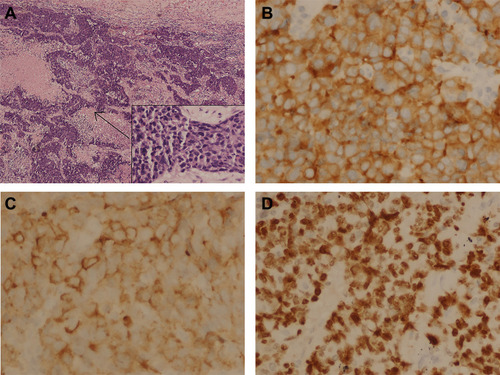
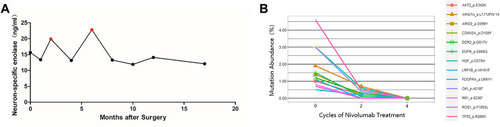
Table 1 Mutations Detected by NGS
Figure 3 Abdominal CT scan of the metastatic tumor in left adrenal gland before and during the treatment course. (A) Metastatic tumor in left adrenal gland before the treatment of nivolumab (Red arrow). (B) Metastatic tumor in left adrenal gland after 4 cycles treatment of nivolumab (Red arrow); (C) Metastatic tumor in left adrenal gland after 8 cycles treatment of nivolumab (Red arrow); (D) Metastatic tumor in left adrenal gland after 12 cycles treatment of nivolumab (Red arrow); (E) Metastatic tumor in left adrenal gland after 15 cycles treatment of nivolumab (Red arrow); (F) Metastatic tumor in left adrenal gland after 3 months since the drug withdrawal (Red arrow); (G) Metastatic tumor in left adrenal gland after 7 months since the drug withdrawal (Red arrow); (H) Metastatic tumor in left adrenal gland after 12 months since the drug withdrawal (Red arrow).
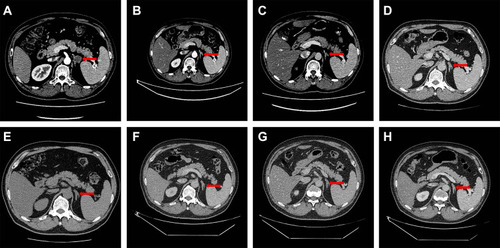
Figure 4 Immunohistochemical staining of primary tumor in lung for PD-L1. (A) Hematoxylin-eosin staining of the tumor tissue (200X). (B) Immunohistochemical staining for PD-L1 (Negative, 200X).
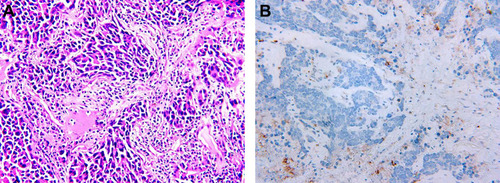
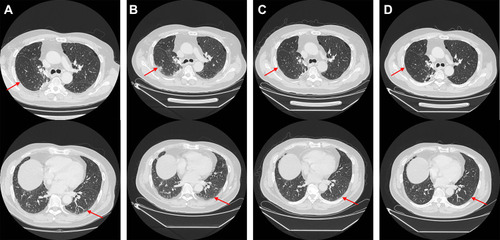
Figure 5 Chest CT scan of the interstitial pneumonia related to nivolumab treatment before and during the treatment course by acetylcysteine and corticosteroids. (A) Interlobular septal thickening and subpleural ground-glass opacities before the 16th cycle of nivolumab treatment (Red arrow). (B) Interlobular septal thickening and subpleural ground-glass opacities got worse in 3 months after nivolumab withdrawal (Red arrow). (C) Interlobular septal thickening and subpleural ground-glass opacities got better in 4 months since acetylcysteine and corticosteroids treatment (Red arrow). D. Interlobular septal thickening and subpleural ground-glass opacities disappeared in 4 months since acetylcysteine and corticosteroids withdrawal (Red arrow).