Figures & data
Figure 1 Downregulation of miR-140 in PC9/R and A549/R. (A) CCK-8 assay was used to test the sensitivity of PC9, PC9/R, A549 and A549/R cells to different concentrations of cisplatin (0~50 μM). (B) IC50 of cisplatin to PC9, PC9/R, A549 and A549/R cells. (C) QRT-PCR analysis was used to evaluate the different of miR-140 expression between routine NSCLC cells and cisplatin-resistant NSCLC cells. *P<0.05.
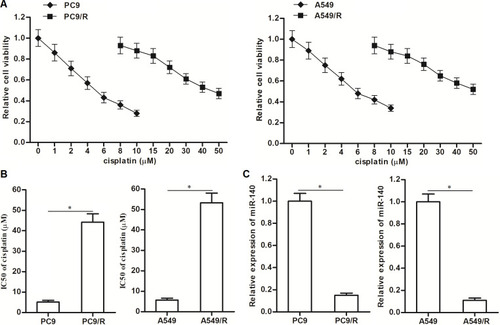
Figure 2 Expression of miR-140 partially determined sensitivity of NSCLC cells to cisplatin. (A) Transfection efficiency of miR-140 mimic in PC9/R and A549/R was evaluated by qRT-PCR analysis. (B) MiR-140 increased the cytotoxicity of cisplatin (10 μM) against PC9/R and A549/R. (C) Effect of miR-140 on reducing the IC50 of cisplatin to PC9/R and A549/R. (D) Transfection efficiency of anti-miR-140 in PC9 and A549 was evaluated by qRT-PCR analysis. (E) Anti-miR-140 decreased the cytotoxicity of cisplatin (10 μM) against PC9 and A549. (F) Effect of anti-miR-140 on increasing the IC50 of cisplatin to PC9 and A549. *P<0.05 vs NCO group. #P<0.05 vs cisplatin+NCO group.
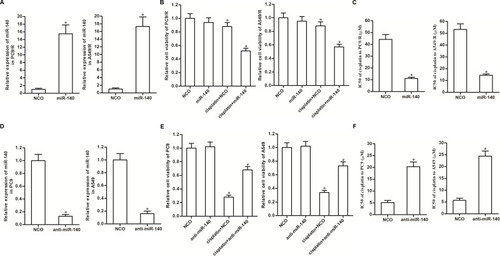
Figure 3 MiR-140 targeted SIRT1 in NSCLC. (A) Putative binding sequence of SIRT1 3’-UTR paired with miR-140. (B) Cisplatin-resistant NSCLC cells expressed higher level of SIRT1 compared to the routine NSCLC cells at the mRNA level. (C) Cisplatin-resistant NSCLC cells expressed higher level of SIRT1 compared to the routine NSCLC cells at the protein level. (D) Transfection with miR-140 mimic decreased the expression of SIRT1 in PC9/R and A549/R. (E) Transfection with anti-miR-140 increased the expression of SIRT1 in PC9 and A549. (F) MiR-140 decreased the luciferase activity of pGL3 reporters which contained wild type SIRT1 3’-UTR. *P<0.05. #P<0.05 vs NCO group.
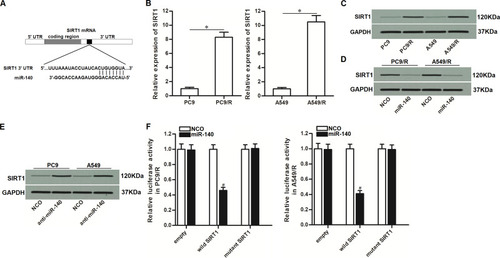
Figure 4 Overexpression of SIRT1 was responsible for the cisplatin resistance of PC9/R and A549/R. (A) Transfection efficiency of SIRT1 plasmid in PC9 and A549 was evaluated by Western blot assay. (B) SIRT1 plasmid decreased the cytotoxicity of cisplatin (10 μM) against PC9 and A549. (C) Transfection efficiency of SIRT1 siRNA in PC9/R and A549/R was evaluated by Western blot assay. (D) SIRT1 siRNA increased the cytotoxicity of cisplatin (10 μM) against PC9/R and A549/R. *P<0.05 vs empty plasmid group. #P<0.05 vs cisplatin+empty plasmid group. &P<0.05 vs control siRNA group. $P<0.05 vs cisplatin+control siRNA group.
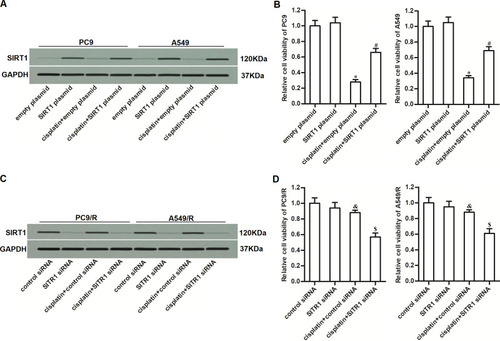
Figure 5 MiR-140 decreased the cisplatin-resistance of PC9/R and A549/R through inhibition of SIRT1. (A) Effect of SIRT1 plasmid on protecting the PC9/R and A549/R cells from the cytotoxicity of co-treatment with cisplatin (10 μM) and miR-140. (B) SIRT1 plasmid reduced the effect of miR-140 on decreasing the IC50 of cisplatin to PC9/R and A549/R. *P<0.05 vs NCO group. #P<0.05 vs cisplatin+NCOgroup. &P<0.05 vs cisplatin+miR-140 group. $P<0.05 vs miR-140 group.
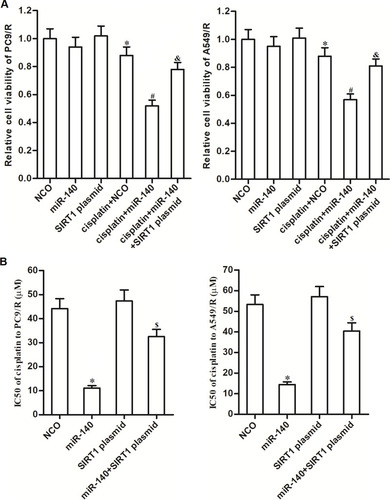
Figure 6 MiR-140 promoted the cisplatin-induced apoptosis through the ROS pathway. (A) Cells which were treated with cisplatin (10 μM), miR-140, SIRT1 plasmid and NAC (2 mM). 48 h later, ROS level of PC9/R and A549/R was evaluated by flow cytometry. (B) Western blot analysis was performed to detect the cleavage of caspase-9 and caspase-3 in PC9/R and A549/R cells which were treated with cisplatin (10 μM), miR-140, SIRT1 plasmid and NAC (2 mM). (C) Cells which were treated with cisplatin (10 μM), miR-140, SIRT1 plasmid and NAC (2 mM). 48 h later, flow cytometry analysis was performed to detect the apoptotic rate of PC9/R and A549/R cells. *P<0.05 vs cisplatin+NCO group. #P<0.05 vs cisplatin+miR-140 group.
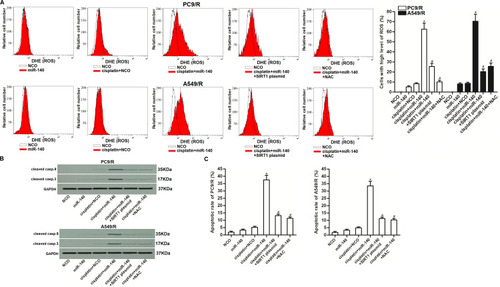
Figure 7 Role of ROS/JNK pathway in miR-140-promoted apoptosis. (A) Expression level of phosphorylated JNK in PC9/R and A549/R cells which were treated with cisplatin (10 μM), miR-140, SIRT1 plasmid, NAC (2 mM) and SP600125 (50 μM). (B) SP600125 (50 μM) failed to change the ROS level in PC9/R and A549/R cells which were co-treated with cisplatin (10 μM) and miR-140 for 48 h. (C) Cytotoxicity of co-treatment with cisplatin (10 μM) and miR-140 against PC9/R and A549/R was inhibited by SIRT1 plasmid, NAC (2 mM) and SP600125 (50 μM). (D) Apoptotic and necrotic rate of NSCLC cells treated with cisplatin (10 μM), SIRT1 plasmid, NAC (2 mM) and SP600125 (50 μM). Cells were treated for 48 h before detection by flow cytometry. *P<0.05 vs NCO group. #P<0.05 vs cisplatin+NCO group. &P<0.05 vs cisplatin+miR-140 group.
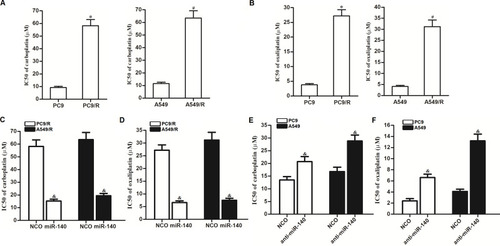
Figure 8 Effect of miR-140 on reversing the cross-resistance of PC9/R and A549/R to carboplatin and oxaliplatin. (A) PC9/R and A549/R exhibited cross-resistance to carboplatin. (B) PC9/R and A549/R exhibited cross-resistance to oxaliplatin. (C) MiR-140 reduced the cross-resistance of carboplatin to PC9/R and A549/R. (D) MiR-140 reduced the cross-resistance of oxaliplatin to PC9/R and A549/R. (E) Knockdown of miR-140 induced the resistance of carboplatin to PC9 and A549. (F) Knockdown of miR-140 induced the resistance of oxaliplatin to PC9 and A549. *P<0.05 vs PC9 group. #P<0.05 vs A549 group. &P<0.05 vs NCO group.