Figures & data
Table 1 Documented Clinicopathological Characteristics of Patients Co-Existing CLL and MDS
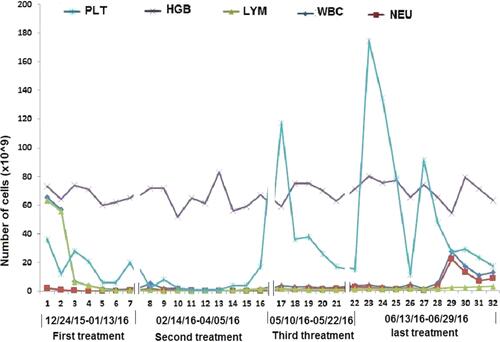
Table 2 Patient Characteristics During the Process
Figure 2 Images A & E, B & F, C & G, and D & H show the initial, second, third and fourth bone marrow (BM) aspirate cells smear.(H & E X400) and biopsy pathological tests (H & E X200) respectively. Cells characters: (A) (12/25/2015) Hematopoietic hyperplasia; Mature lymphocytes at 87%; Background trilineage hematopoiesis was reduced with normal morphology (E) (12/25/2015) Hematopoietic area at 90%. Abnormal cells increased in number, containing a large cell body, a moderate amount of cytoplasm, round or oval nucleus. MF-1 level. (B) (03/31/2016) Hematopoietic hyperplasia;Mature lymphocytes at 21%. Iron stain positive Sideroblasts were 35% of all normoblasts. Erythroid series revealed numerous proerythroblasts and basophilic erythroblast (56%), with abnormal nucleus easily observed (<10%). Myeloid and megakaryocyte were reduced. (F) BM biopsy (03/31/2016) demonstrated Hematopoietic area at 90%. A marked increase in granulocyte and immature erythroid cell and small megakaryocytes. Immunohistochemistry showed CD42b megakaryocytes(+); CD34(-);CD117, CD5, MPO, CD20andCD23 (scattered or clustered+). MF-1 level. (C) (05/11/2016) Hematopoietic hyperplasia Mature lymphocytes at 15%. Sideroblasts were 10% of all normoblasts. Erythrocytes were active. Parts of erythroblasts were megaloblastic (<10%). Myeloid hyperplasia was reduced. (G) (05/11/2016) BM biopsy showed increased fibrosis, and granulocyte, erythrocyte, and megakaryocyte cells were easily observed. Lymphocytes were focally or dispersedly distributed. Immunohistochemistry revealed abnormal B cells had increased in quantity, reacting positively for CD20, CD5, CD23, and 42b megakaryocytes, CD34 (<2%), CD117 (a small amount), MPO (a small amount), but negative for CD3 Remarkable fibrous proliferation (MF-2 level). Small megakaryocytes were easy to see. Immunohistochemistry revealed the number of abnormal B cells increased, positive for CD20, CD5, CD23; CD42b megakaryocytes(+); CD34 (<2%); CD117 (a small amount); MPO (a small amount); CD3(-). (D) (06/13/2016) Hematopoietic area at 80%. A significant increase in the amount of large body cells with abundant cytoplasm, round or slightly irregular nucleus, thicker nuclear chromatin, and prominent nucleoli, IHC showed negative for CD20, PAX5, CD3, CD138, CD117, CD42b, Ckpan, Cam5.2. Small lymphocytes increased in number and were positive for CD20, PAX5, CD5 and CD23. (H) BM biopsy showed Lymphocytes taking up 62.71% of all nucleated cells, with abnormal small mature B lymphocyte at 53.22%, positive for CD19, CD20 (a small amount), CD22, CD23, Lambda, and negative for CD10, FMC7, CD34 and Kappa with light chain restriction. Juvenile erythrocyte and granulocyte had reduced.
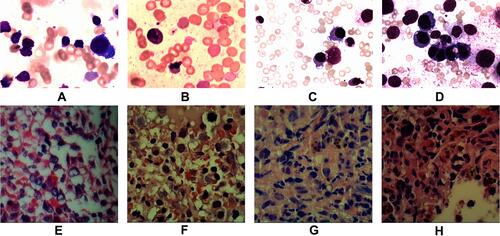
Figure 3 A, B, C, and D represent the initial, second, third and fourth results taken during conventional cytogenetic analysis of bone marrow. RHG-banded metaphases were analysed and karyotyped according to ISCN (2013). (A) Karyotype on 25 December 2015, LTSC showing:66~68, XY,+Y,+1,+1,+2,+3,+3,+4,+6,+6,+7, +8,+9, +11,+12, +13,+15, +16,+17,+21, +21,+21[cp10]/46, XY[3]. (B) Karyotype on 1 April 2016, USSTC showing less cells with poor proliferation: 46, XY[8]. (C) Karyotype on 11 May 2016, LTSC showing 55~65, XY,+Y, +1,+2, +2,+4, +5,+5,+5,+8, t(9;22)(q24; q11.2), +13,+14, +14,+15, +16, +17,+20,+21,+der(22)t(9;22)[cp14]/46, XY[6]. (D) Karyotype on 14 June 2016, LTSC showing 57~59, XY,+Y,+1,+2,+3,+6,+8, add(11)(p15), +13,+15,+16,+19,+21,+21, +21[cp20].
![Figure 3 A, B, C, and D represent the initial, second, third and fourth results taken during conventional cytogenetic analysis of bone marrow. RHG-banded metaphases were analysed and karyotyped according to ISCN (2013). (A) Karyotype on 25 December 2015, LTSC showing:66~68, XY,+Y,+1,+1,+2,+3,+3,+4,+6,+6,+7, +8,+9, +11,+12, +13,+15, +16,+17,+21, +21,+21[cp10]/46, XY[3]. (B) Karyotype on 1 April 2016, USSTC showing less cells with poor proliferation: 46, XY[8]. (C) Karyotype on 11 May 2016, LTSC showing 55~65, XY,+Y, +1,+2, +2,+4, +5,+5,+5,+8, t(9;22)(q24; q11.2), +13,+14, +14,+15, +16, +17,+20,+21,+der(22)t(9;22)[cp14]/46, XY[6]. (D) Karyotype on 14 June 2016, LTSC showing 57~59, XY,+Y,+1,+2,+3,+6,+8, add(11)(p15), +13,+15,+16,+19,+21,+21, +21[cp20].](/cms/asset/d67bda0d-8fbc-46a0-8450-ca1a22bb6c47/dott_a_12175806_f0003_b.jpg)