Figures & data
Figure 1 Contrast-enhanced, axial, T1-weighted magnetic resonance images of the tumor.
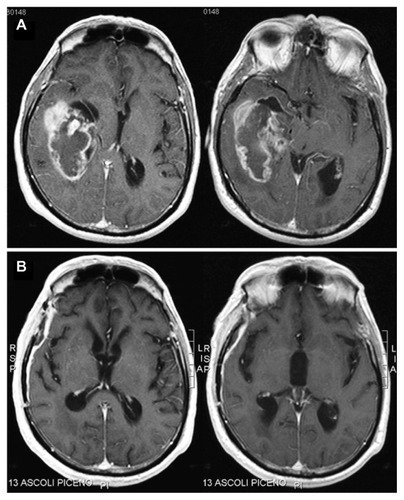
Figure 2 The patient’s third ventricle nodular lesion.
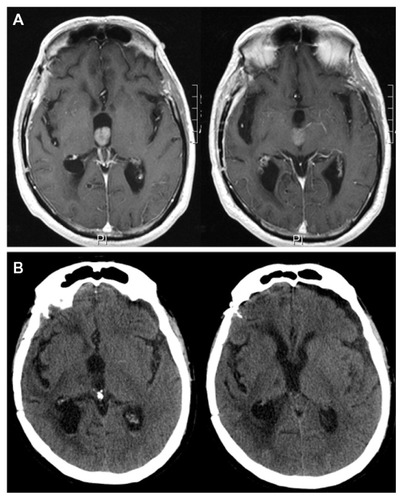
Figure 3 Close-up endoscopic third ventricle views.
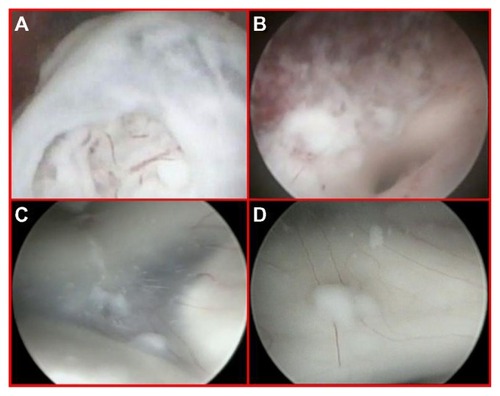
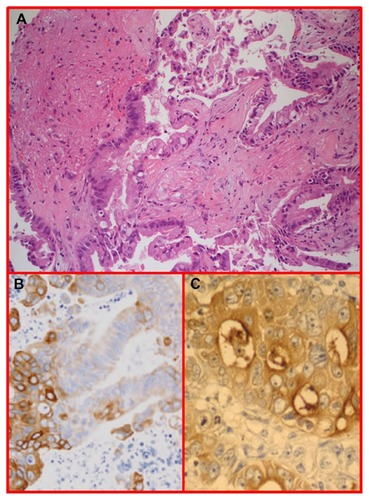
Figure 4 H and E glioblastoma multiforme, World Health Organization grade IV, with evidence of nuclear pleomorphism, mitotic activity, endothelial hyperplasia, and areas of palisading necrosis.
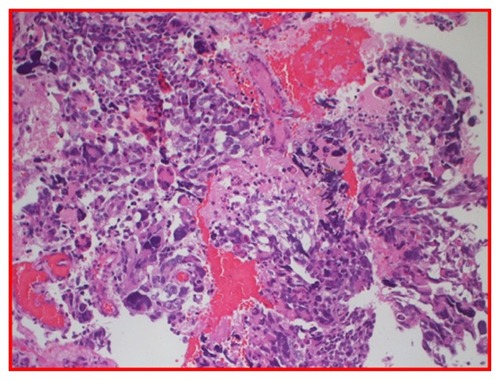
Figure 5 Preoperative, contrast-enhanced, axial, T1-weighted magnetic resonance images.
*The left ventricle looked normal.
Abbreviation: MRI, magnetic resonance imaging.
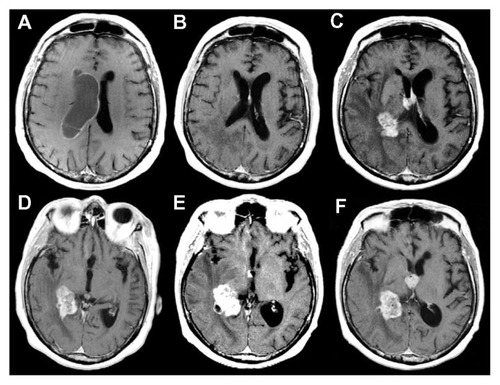
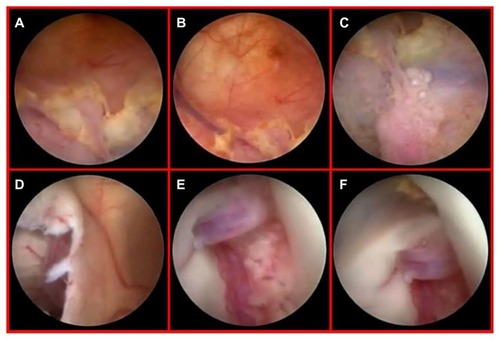
Figure 6 Diffuse yellowish coloration of the walls of the right lateral ventricle discovered during neuroendoscopic examination, with clusters of neoplastic cells embedding the choroid plexus and a thin sheet of tumor cells forming small nodules in the subependymal region (A–C). Endoscopic view of the septostomy with a contralateral apparently normal ependymal layer and choroid plexus (D and E). Close-up view of an initial, contralateral tumor infiltration evident through the fibers of the subependymal layer, situated along the anterior commissure (F). This finding was undetected by preoperative magnetic resonance imaging (see ).