Figures & data
Figure 1 Effect of ulinastatin (UTI) or curcumin (CUR) on cell viability and proliferation of HCT-116. (A) Cytotoxicity of UTI against HCT-116 cells in vitro. Tumor cells (5.0 × 10Citation3) in each well of a 96-well culture plate were incubated for 24 hours at 37°C with or without various concentrations of UTI. CCK-8 was added to each well and, after incubation for 2 hours, the absorbance was measured at 450 nm. (B) HCT-116 cells were treated with different concentrations of CUR for 24 hours, and cell viability was measured using the CCK-8 method. Concentrations of CUR resulting in 50% growth inhibition were indicated as individual IC50 (50% cell growth inhibitory concentrations) values.
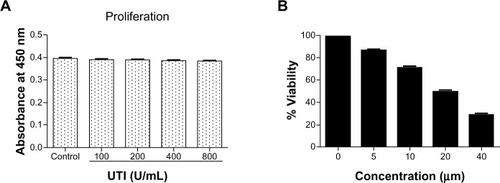
Figure 2 Effect of ulinastatin (UTI) and curcumin (CUR) on cell migration and invasion. (A) Migration of HCT-116 was assayed by wound healing assay. Cells were cultured to nearly confluent cell monolayer. A scratch wound was created on the cell surface using a micropipette tip. The monolayer was washed with phosphate buffered saline, and then UTI (800 U) or CUR (10 μM) was added or not. The cultures were incubated at 37°C for 0 hours, 24 hours, and 48 hours, respectively, and pictures were taken using light microscopy (×100). (B) The width of the wound was measured and the wound closure rate was calculated. (C) Transwell in vitro invasion assay detects the effect of UTI and CUR on the invasive ability of colon cancer cells. (a) cells treated with PBS; (b) cells treated with UTI; (c) cells treated with CUR; (d) cells treated with UTI and CUR. (D) The invaded cell numbers were measured and compared.
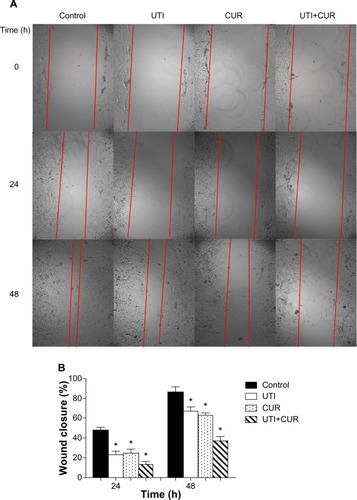
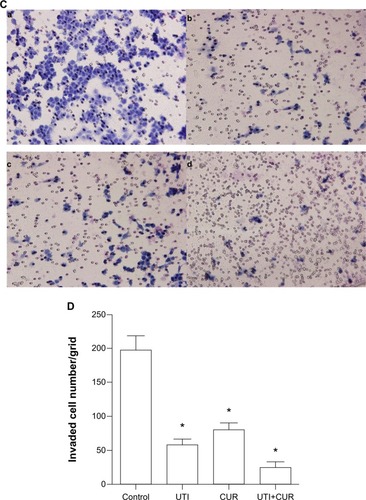
Figure 3 Measurement of bioluminescence in HCT-116-Luc-GFP cells. HCT-116-Luc-GFP cells were counted with a hemocytometer and plated in a 96-well plate at various concentrations. The plate was imaged to verify that the cells bioluminesced in a concentration-dependent manner. (A) Quantitation of bioluminescence (photons/sec) was graphed. (B) Linear regression analysis showed a good correlation between cell number and mean bioluminescence imaging or fluorescent intensity (rCitation2=0.9978).
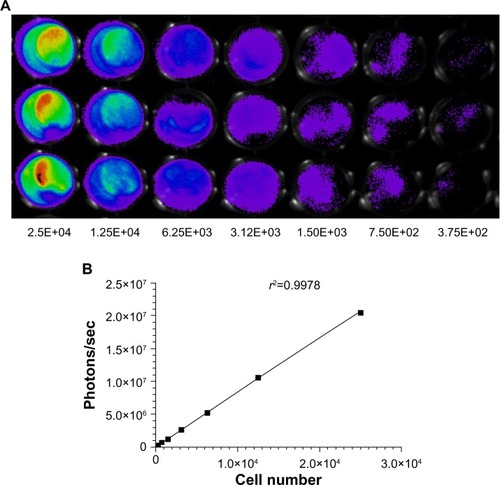
Figure 4 Ulinastatin (UTI) and curcumin (CUR) inhibits liver metastasis and prolongs survival. (A) Luciferase-expressing HCT-116 cells were injected into the spleens of BLAB/c mice. The control group (n=7) received vehicle, the UTI group (n=7) was injected with UTI at 8,000 U/mouse once daily, the CUR group (n=7) was administered orally CUR alone (1 g/kg) once daily, and the UTI plus CUR group (n=7) was treated with a combination of UTI (8,000 U/mouse) and CUR (1 g/kg). Therapy was continued for 4 weeks. Bioluminescence imaging was used to monitor liver metastasis of HCT-116-Luc-GFP cells in vivo 1 day and 28 days after splenic injection. (B) Survival of mice treated with/without UTI and/or CUR was assayed after injecting HCT-116-Luc-GFP cells.
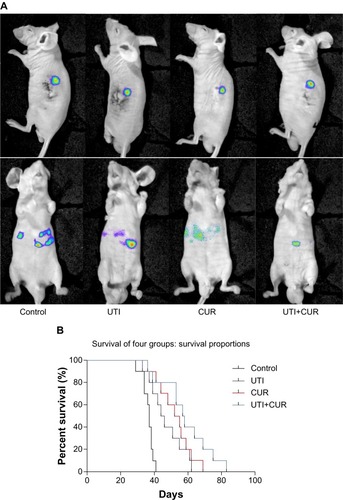
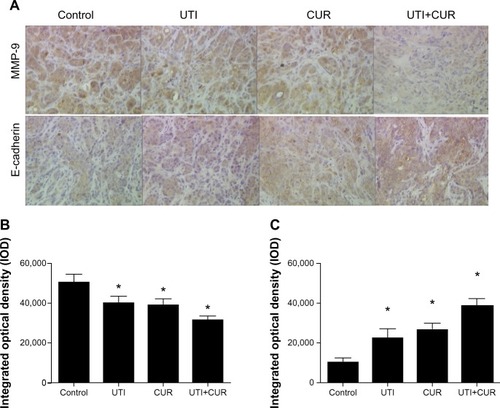
Figure 5 Expression of MMP-9 and E-cadherin in metastatic tumors was detected by immunohistochemical assay (original magnification 400×). (A) Expression of MMP-9 and E-cadherin in four groups. Differences in MMP-9 (B) and E-cadherin (C) protein expression are shown.
Abbreviations: CUR, curcumin; MMP-9, modulating matrix metalloproteinase-9; UTI, ulinastatin.